 | Facilitated Transport across the Barrier
Carrier systems appear to be involved in the transport of several materials across the barrier. Glucose, ions, and certain amino acids utilize this type of system. The carrier system for glucose is stereospecific as n-glucose, but not Lglucose, is readily transported into the brain. Lactic, pyruvic, and acetic acids also utilize such carriers.
While proteins are virtually excluded from the brain, certain amino acids pass readily into it. Included are the essential amino acids and those which are precursors for the production of neurotransmitters. The latter include tyrosine (required for norepinephrine and dopamine synthesis) and tryptophan (for serotonin synthesis). Similarly, neuroactive peptides whose amino acid sequences have been clearly identified such as substance P, methionine enkephalin and leucine enkephalin, ,B-endorphin, ACTH, angiotensin II, oxytocin, vasopressin, somatostatin, thyrotropin-releasing factor, and luteinizing hormone-releasing factor rely on a steady transport of these amino acids from plasma to brain for their continued synthesis.
Ions cross the barrier into brain but do so much more slowly than into other body tissues. An intravenous K + administration exchanges much more quickly with muscle tissue that it does with brain. Ca2+ and Mg2+ transport is equally slow, while N a + is somewhat faster. H+ ion transport into the brain is very slow.
Certain areas of the brain apparently contain no blood-brain barrier. These include the neurohypophysis, median eminence of the hypothalamus, the area postrema, and the pineal gland. Because many circulating hormones control their own release through negative feedback to the hypothalamus, the importance of barrier lack in this area is readily apparent. If such hormones are to influence the hypothalamic output of releasing or inhibiting factors to the anterior pituitary via the hypothalamohypophyseal portal system, they must not be barred from the hypothalamus by a barrier system. Similarly, osmoreceptors of the hypothalamus must be able to constantly and easily detect changes in the osmolality of the plasma if the release of antidiuretic hormone (ADH) is to proceed properly.
CEREBRAL BLOOD FLOW AND OXYGEN CONSUMPTION
 | CEREBRAL BLOOD FLOW
The average cerebral blood flow in humans is approximately 55 mL per 100 g of brain tissue per minute. This is a little over 700 mL/min for a 1350-g brain. Thus while the human brain comprises only about 2.5 percent of the body's weight, it receives almost 15 percent of the cardiac output, attesting to the high vascular demands of this organ.
A reliable and frequently used method of determining cerebral blood flow is the method of Kety and Schmidt. It is based on the Fick principle and utilizes the arteriovenous difference of a freely diffusible gas such as N2O as it passes through the brain. Accordingly, the flow of blood through the brain can be determined by measuring the amount of N2O removed from the blood by the brain per minute and dividing this by the arteriovenous difference of N2O as it passes through the brain. The cerebral blood flow is higher in children than in adults, typically exceeding 100 mL per 100 g per minute. However, contrary to popular thinking, the blood flow decreases only slightly with advancing age. The brain utilizes fully 25 percent of the body's total oxygen consumption. The arteriovenous O2 difference is relatively high since the brain receives only 15 percent of the cardiac output. The arteriovenous difference is 6.6 mL per 100 ml., falling from 19.6 to 13 mL per 100 mL as blood passes through the brain (Fig. 17-2). Thus we can calculate a cerebral oxygen consumption of approximately 3.5 mL per 100 g per minute. This value is greater in skeletal muscle, skin. and liver. but less in cardiac muscle and kidney.
|
 |
OXYGEN CONSUMPTION
The utilization of oxygen by the brain is not uniform throughout its mass. The gray matter consumes as much as 94 percent of cerebral oxygen. while the white matter, which makes up fully 60 percent of the brain's mass. consumes only 6 percent. Oxygen consumption, and hence oxygen need, increases as we move up the neuraxis. It is lowest in the spinal cord and increases through the medulla. midbrain, thalamus, cerebellum, and cerebral cortex. Thus it is not surprising to find that the sensorimotor functions of the cerebral cortex are more sensitive to hypoxic damage than are the vegetative functions of the pontomedullary areas. Progressive decreases in cerebral oxygen consumption are always accompanied by progressive decreases in the level of mental alertness. Compared to the mentally alert young man with an O2 consumption of 3.5 mL per 100 g per minute, the mentally confused states associated with diabetic acidosis, insulin hypoglycemia, and some forms of cerebral arteriosclerosis might typically show O2 consumptions rates down to 2.8 mL per 100 g per minute. Finally, the comatose states of diabetic coma. insulin coma. and anesthesia can show consumption rates as low as 2.0 mL per 100 g per minute. On the other hand. O2 consumption by the brain increases during convulsions.
|
 | EFFECTS OF OXYGEN DEPRIVATION
Almost all of the oxygen consumed by the brain is utilized for the oxidation of carbohydrate. Sufficient energy is released from this process so that the normal level of oxygen utilization is adequate to replace the 12 mmol or so of A TP which the whole brain uses per minute. However, since the normal brain reserve of A TP and creatine phosphate (CrP) totals only about 8 rnmol, less than a minute's reserve of high energy phosphate bonds is actually available if production were to suddenly stop. In the absence of oxygen, the anerobic glycolysis of glucose and glycogen could supply only another 15 mmol of A TP, as these two energy substrates are stored in such low quantities in brain tissue.
A continuous uninterrupted supply of oxygen to the brain is essential in order to maintain its metabolic functions and to prevent tissue damage. The oxygen-independent glycolytic pathway (anerobic glycolysis) is insufficient, even at maximum operating levels, to supply the heavy demands of the brain. Thus a loss of consciousness occurs when brain tissue P02 levels fall to 15 to 20 mmHg. This level is reached in less than 10 s when cerebral blood flow is completely stopped
Low tissue oxygen levels in the brain (hypoxidosis) can be caused by decreased blood flow (ischemia) or with adequate blood flow accompanied by low levels of blood oxygen (hypoxemia). It is important to recognize that decreased P02 caused by ischemia is accompanied by decreased brain glucose and increased brain CO2 while hypoxemia with normal blood flow is not accompanied by changes in brain glucose or CO2, with complete cessation of CBF, irreversible damage occurs to brain tissue within a few minutes and the histological effects observed are remarkably similar whether caused by ischemia, hypoxemia, or hypoglycemia.
Experimental studies on rats and mice in which arterial P02 is progressively reduced have illustrated some aspects of hypoxemia which are likely to be similar in humans. A drop in arterial P02 to 50 mmHg (normal, 96 mmHg) produces no change in CBF, O2 utilization by the brain, or lactic acid production. However, as P02 levels drop to 30 mmHg, a 50 percent increase in CBF is observed along with the onset of coma, decreased oxygen utilization, and increased lactic acid production. When the P02 drops further to 15 mmHg, 50 percent of the animals die because of cardiac failure. The remainder show a tremendous increase in lactic acid production, but, surprisingly, levels of ATP, ADP, and AMP remain normal. If cerebral perfusion is artificially maintained while the arterial P02 is decreased further, ATP, ADP, and AMP levels still remain normal. The implication is that the coma observed at low oxygen levels may not be due to a decrease in ATP but instead to some still unexplained mechanism. It appears likely that cardiac complications caused by hypoxemia and the subsequent effect on cerebral blood flow may actually be a primary cause of the irreversible pathologic damage to the brain.
Hypoxia, such as that brought on by high altitudes, brings on a number of symptoms, including drowsiness, apathy, and decreases in judgment. Unless oxygen is administered within half a minute or so, coma, convulsions, and depression of the EEG occur.
|
GLUCOSE METABOLISM
 Glucose metabolism Glucose metabolism
Glucose is virtually the only energy substrate which the brain can use. Free fatty acids, used by most other tissues when glucose is in short supply, are excluded from the brain by the blood-brain barrier. The brain extracts 6.6 mL of O2 from each 100 mL of cerebral blood and returns 6.7 mL of CO2. Thus the respiratory quotient (RQ) of the brain is approximately 1.0, indicating carbohydrate utilization only. Brain glucose consumption is normally about 10 mg per 100 ml., accounting for almost 75 percent of the liver's production and further attesting to the brain's heavy dependence on glucose. Adenosine triphosphate (ATP), produced by the metabolic degradation and oxidative phosphorylation of glucose, is the useful energy currency in brain tissue. About 85 percent of the circulating glucose extracted from the cerebral arterial blood is converted to CO2 via the tricarboxylic acid (TCA) cycle, while 15 percent is converted to lactic acid. The general scheme for glucose metabolism in the brain is similar to that in other tissues and is illustrated in Fig.1. The enormous ATP requirements of the brain are partly due to neurotransmitter synthesis, release, and reuptake as well as intracellular transport and complex synthetic mechanisms. But undoubtedly the greatest percentage of ATP is utilized to power the ion pumps which restore membrane potentials, enabling neurons to maintain their excitability.
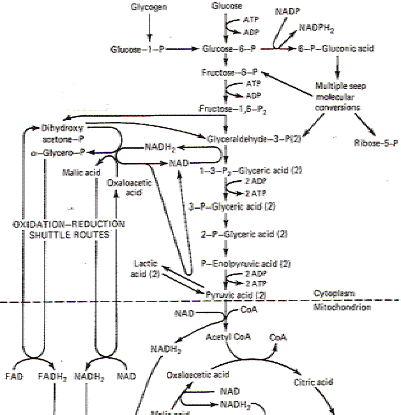
Fig-1
 EFFECTS OF GLUCOSE DEPRIVATION EFFECTS OF GLUCOSE DEPRIVATION
In the healthy normal functioning brain, glucose is the only substrate utilized for energy metabolism. Thus hypoglycemia presents the brain with a very serious problem. While most other tissues can shift to utilizing free fatty acids (FFA) as an alternative energy source when glucose is lacking, the brain cannot because they are excluded by the blood-brain barrier. While there is some evidence that the brain can utilize β-hydroxybutyric acid for energy metabolism when glucose levels are low or when fats are being mobilized for energy metabolism throughout the rest of the body, the brain could never supply its high energy demands by this method alone in the absence of glucose. Thus the brain is dependent on an uninterrupted supply of blood-borne glucose to energize its cells.
Decreases in blood glucose bring on disturbances in cerebral function. Depending on the level of hypoglycemia, these changes range from mild sensory disturbances to coma. At blood glucose levels of 19 mg per 100 mL or below (normal is 60 to 120 mg per 100 mL), a mentally confused state occurs. Brain O2 utilization falls to 2.6 mL per 100 g per minute (normal, 3.5 mL per 100 g per minute) and glucose utilization drops as well. Coma commences when glucose levels fall to 8 mg per 100 mL.
Epinephrine can be effective in reversing the effects of hypoglycemia by promoting liver glycogenolysis. However, attempts to solve the problem by substituting other carbohydrate metabolic substrates have been largely unsuccessful, with the single exception of mannose. This is the only monosaccharide other than glucose which the brain appears to utilize directly. It crosses the blood-brain barrier and directly replaces glucose in the glycolytic pathway. However, its normal level in the blood is too low to be of any real help in reversing the cerebral effects of hypoglycemia. Unless reversed quickly, comatose levels of prolonged hypoglycemia will bring on necrosis of cerebrocortical cells and (to a lesser extent) other brain regions as well.
NEUROACTIVE CHEMICALS
Neurons in the CNS produce a large number of special molecules which function as neurotransmitters or are suspected to do so, including acetylcholine (ACh), norepinephrine (NE), dopamine (DA), y-aminobutyric acid (GABA), aspartic acid, glutamic acid, glycine, and substance P. CNS neurons also synthesize a number of neuropeptides which perform quite specific endocrine roles. We will take a closer look at these neuroactive chemicals now.
 Acetylcholine Acetylcholine
 Acetylcholine has long been recognized as an important neurotransmitter. It's released by preganglionic sympathetic and parasympathetic nerve fiber terminals as well as postganglionic parasympathetic and certain select sympathetic fibers. It is also the only recognized neurotransmitter at the skeletal muscle neuromuscular junction. Unfortunately we don't have nearly as complete a picture of the distribution of cholinergic neurons in the CNS. There appear to be cholinergic fibers associated with the arousal or activating systems of the brain which project from the midbrain reticular formation, hypothalamus striatum, and septum to the neocortex. ACh and the enzymes necessary for its synthesis are also found in the hippocampus, corpus striatum, and retina. Acetylcholine has long been recognized as an important neurotransmitter. It's released by preganglionic sympathetic and parasympathetic nerve fiber terminals as well as postganglionic parasympathetic and certain select sympathetic fibers. It is also the only recognized neurotransmitter at the skeletal muscle neuromuscular junction. Unfortunately we don't have nearly as complete a picture of the distribution of cholinergic neurons in the CNS. There appear to be cholinergic fibers associated with the arousal or activating systems of the brain which project from the midbrain reticular formation, hypothalamus striatum, and septum to the neocortex. ACh and the enzymes necessary for its synthesis are also found in the hippocampus, corpus striatum, and retina.
 Acetylcholine is formed by the reaction of choline with acetyl coenzyme A (acetyl CoA) in the presence of the enzyme choline acetyltransferase (CAT). Since neurons can't synthesize choline, the ultimate source of choline for ACh synthesis is the choline of the plasma. Acetyl CoA is synthesized within presynaptic cytoplasm by the A TP-energized reaction of acetate with CoA. Once ACH has been synaptically released and has produced its postsynaptic effects on membrane permeability, it is hydrolyzed within microseconds by the enzyme acetylcholinesterase (AChE). Interestingly enough, while negligible amounts of ACh are reabsorbed by presynaptic terminals in the peripheral nervous system (hydrolysis by AChE being overwhelmingly dominant), its reuptake by the terminals in brain is considerable. Nevertheless, its failure to be resequestered into synaptic vesicles leaves the significance of this process in doubt. Acetylcholine is formed by the reaction of choline with acetyl coenzyme A (acetyl CoA) in the presence of the enzyme choline acetyltransferase (CAT). Since neurons can't synthesize choline, the ultimate source of choline for ACh synthesis is the choline of the plasma. Acetyl CoA is synthesized within presynaptic cytoplasm by the A TP-energized reaction of acetate with CoA. Once ACH has been synaptically released and has produced its postsynaptic effects on membrane permeability, it is hydrolyzed within microseconds by the enzyme acetylcholinesterase (AChE). Interestingly enough, while negligible amounts of ACh are reabsorbed by presynaptic terminals in the peripheral nervous system (hydrolysis by AChE being overwhelmingly dominant), its reuptake by the terminals in brain is considerable. Nevertheless, its failure to be resequestered into synaptic vesicles leaves the significance of this process in doubt.
Acetate + CoA + ATP ~ acetyl CoA + AMP + 2Pi
 Catecholamines Catecholamines
 The catecholamine neurotransmitters are norepinephrine (NE) and dopamine (DA). The synthesis of both of these amines proceeds from the amino acid tyrosine (Fig. 17-5). Tyrosine is converted to 3,4-dihydroxyphenylalanine (dopa) by the enzyme tyrosine hydroxylase. Subsequent decarboxylation by dopa decarboxylase converts dopa to 3,4-dihydroxyphenylethylamine (dopamine). This is as far as the synthesis proceeds in dopaminergic neurons. In norepinephrinergic neurons, an additional step converts dopamine to norepinephrine by action of the enzyme dopamine ,B-hydroxylase. The catecholamine neurotransmitters are norepinephrine (NE) and dopamine (DA). The synthesis of both of these amines proceeds from the amino acid tyrosine (Fig. 17-5). Tyrosine is converted to 3,4-dihydroxyphenylalanine (dopa) by the enzyme tyrosine hydroxylase. Subsequent decarboxylation by dopa decarboxylase converts dopa to 3,4-dihydroxyphenylethylamine (dopamine). This is as far as the synthesis proceeds in dopaminergic neurons. In norepinephrinergic neurons, an additional step converts dopamine to norepinephrine by action of the enzyme dopamine ,B-hydroxylase.
 The enzymatic degradation of the two catecholamines is illustrated in Fig. 17-6. Catechol-o-methyltransferase (COMT) and monoamine oxidase (MAO) produce inactive products which have little effect on receptor sites. MAO catalyzes the oxidative deamination of norepinephrine to 3,4-dihydroxymandelic acid and dopamine to 3,4-dihydroxyphenylacetic acid. These products are then methylated by COMT to 3-methoxy-4-hydroxymandelic acid and homovanillic acid, respectively. Alternatively, norepinephrine can first be methylated to normetanephrine and then deaminated to 3-methoxy-4-hydroxymandelic acid. The enzymatic degradation of the two catecholamines is illustrated in Fig. 17-6. Catechol-o-methyltransferase (COMT) and monoamine oxidase (MAO) produce inactive products which have little effect on receptor sites. MAO catalyzes the oxidative deamination of norepinephrine to 3,4-dihydroxymandelic acid and dopamine to 3,4-dihydroxyphenylacetic acid. These products are then methylated by COMT to 3-methoxy-4-hydroxymandelic acid and homovanillic acid, respectively. Alternatively, norepinephrine can first be methylated to normetanephrine and then deaminated to 3-methoxy-4-hydroxymandelic acid.
 Distribution of Norepinephrinergic Fibers Distribution of Norepinephrinergic Fibers
The distribution of norepinephrinergic fibers in the peripheral nervous system is limited to the majority of postganglionic sympathetic neurons. Norepinephrine-releasing neurons in the central nervous system have their cell bodies located in the midbrain, pons, and medulla, primarily in the reticular formation. Two norepinephrine systems are often described in the mammalian brain: the locus ceruleus system and the lateral tegmental system. The cell bodies of the former are located in the locus ceruleus, a prominent nucleus in the brain stem reticular formation at the level of the isthmus. This nucleus is composed entirely of norepinephrinergic neurons. Their fibers project to the spinal cord, brainstern, cerebellum, hypothalamus, thalamus, basal telencephalon, and the entire neocortex. The lateral tegmental system includes those norepinephrinergic neurons with cell bodies located in the dorsal motor nucleus of X, the nucleus of the solitary tract, and the adjacent and lateral tegmentum. The fibers of this system project to the spinal cord, brainstem, hypothalamus, thalamus, and basal telencephalon
 Distribution of Dopaminergic Fibers Distribution of Dopaminergic Fibers
Dopaminergic systems in the CNS are more complex, numerous, and diversely distributed than norepinephrine systems. Seven _d_<?fla..!Jl:~I1.e~gj£ systems can be identified III the mammalian brain.
Nigrostriatal System The neurons in this system project from the pars compacta of the substantia nigra and the mediolateral tegmentum to terminate in the caudate nucleus, putamen, and globus pallidus. A marked reduction in dopamine content in the neostriatum (caudate and putamen) is characteristic in patients with Parkinson's disease. There is good evidence that the dopaminergic neurons of the substantia nigra inhibit their target cells in the caudate nucleus.
Mesocortical System This system is composed of fibers from the substantia nigra and medioventral tegmentum which do not project to the basal nuclei. The fibers terminate in both the neocortex and allocortex. Terminations in the former include the mesial frontal, anterior cingulate, entorhinal, and perirhinal regions. Terminations in the allocortex include the olfactory bulb, anterior olfactory nucleus, olfactory tubercle, piriform cortex, septal area, and amygdaloid complex.
Tuberohypophyseal System These fibers originate in the arcuate and periventricular hypothalamic nuclei, and project to the neurointermediate lobe of the pituitary gland as well as the median eminence. One function of this system appears to be the inhibition of pituitary prolactin secretion. The pathway to the intermediate lobe may serve to inhibit melanocyte-stimulating hormone (MSH) secretion.
Retinal System The dopaminergic neurons of this system are the interplexiform cells of the retina which terminate in both the inner and outer plexiform layers of the retina.
Incertohypothalamic System These fibers project from the zona incerta and the posterior hypothalamus to the dorsal hypothalamic area and the septum. They may playa role in neuroendocrine regulation.
Periventricular System The cell bodies of these fibers are located in the medulla in the area of the dorsal motor nucleus of X, the nucleus of the solitary tract, and the periaqueductal and periventricular gray matter. They terminate in the periaqueductal and periventricular gray, tegmentum, tectum, thalamus, and hypothalamus. Their function is unknown.
Olfactory Bulb System This system is composed of the periglomerular cells of the olfactory bulbs which terminate on the mitral cells of the glomeruli. Their function is unknown.
 Serotonin and Melatonin Serotonin and Melatonin
 Serotonin and melatonin are neuroactive indolealkylamines. Serotonin functions as a CNS neurotransmitter while melatonin, formed by a two-step process from serotonin, may playa hormonal role in the pineal gland. The highest concentration of serotonin anywhere in the body is in the pineal gland. The next highest concentration is in the raphe nuclei of the lower brainstem. The French neurophysiologist Jouvet demonstrated the role of these serotonergic raphe neurons by performing experiments on cats. He selectively destroyed the raphe neurons, producing a significant reduction in brain serotonin levels, and found that the cats became totally insomniac. He followed this by administering p-chlorophenylalanine to another group of cats. This drug, which prevents the conversion of tryptophan to 5-hydroxytryptophan by interfering with the action of the enzyme tryptophan hydroxylase, decreases the raphe concentration of serotonin, because 5-hydroxytryptophan is a serotonin precursor. This group of cats also became insomniac. Subsequent administration of 5-hydroxytryptophan reversed the insomnia, putting the cats to sleep. Serotonin and melatonin are neuroactive indolealkylamines. Serotonin functions as a CNS neurotransmitter while melatonin, formed by a two-step process from serotonin, may playa hormonal role in the pineal gland. The highest concentration of serotonin anywhere in the body is in the pineal gland. The next highest concentration is in the raphe nuclei of the lower brainstem. The French neurophysiologist Jouvet demonstrated the role of these serotonergic raphe neurons by performing experiments on cats. He selectively destroyed the raphe neurons, producing a significant reduction in brain serotonin levels, and found that the cats became totally insomniac. He followed this by administering p-chlorophenylalanine to another group of cats. This drug, which prevents the conversion of tryptophan to 5-hydroxytryptophan by interfering with the action of the enzyme tryptophan hydroxylase, decreases the raphe concentration of serotonin, because 5-hydroxytryptophan is a serotonin precursor. This group of cats also became insomniac. Subsequent administration of 5-hydroxytryptophan reversed the insomnia, putting the cats to sleep.
 Melatonin is formed from serotonin in the pineal gland by acetylization to n-acetyl serotonin by 5_hydroxytryptamin~-n-acetylase. The enzyme 5hydroxyindole-o-methyl transferase then completes the conversion to melatonin. The synthesis of both serotonin and melatonin, as well as the degradation of serotonin, are illustrated in Fig. 17-7 Melatonin is formed from serotonin in the pineal gland by acetylization to n-acetyl serotonin by 5_hydroxytryptamin~-n-acetylase. The enzyme 5hydroxyindole-o-methyl transferase then completes the conversion to melatonin. The synthesis of both serotonin and melatonin, as well as the degradation of serotonin, are illustrated in Fig. 17-7
AMINO ACID NEUROTRANSMITTERS
 | Introduction
Several amino acids have been implicated as neurotransmitters in the CNS, including γ-aminobutyric acid (GABA), glutamic acid, glycine, and aspartic acid. Of these, we know the most about the role of GABA. It was the first amino acid to be established as a neurotransmitter in vertebrate and invertebrate nervous systems. GABA is synthesized in nervous tissue by the alpha decarboxylation of glutamic acid in the presence of glutamic acid decarboxylase (Fig. 17-8).
GABA has usually been described as an inhibitory neurotransmitter and may function primarily in this role in the CNS. It is unusual among amino acids in that it is produced almost exclusively in the brain and spinal cord. Its importance is evidenced by its wide distribution, which has been estimated to include up to one-third of all CNS synapses. The possibility exists that all of the inhibitory cells of the cerebellar cortex are "GABAergic." This includes the Purkinje, stellate, basket, and granular cells. G ABA is also suspected to operate as an inhibitory neurotransmitter in the cerebral cortex, lateral vestibular nucleus, and spinal cord. Chemical analysis has also established the presence of G ABA in the colliculi, diencephalon, and to a lesser extent, the pons, medulla, and much of the cerebral cortex. GABA produces inhibition by hyperpolarizing membranes through increased CI- and K + ion conductance. Glycine, another amino acid transmitter, is also suspected to be inhibitory through the same mechanism. Interestingly enough, glutamic acid, the GABA precursor which chemically differs from it by having two rather than one carboxyl groups, is considered to be an excitatory rather than an inhibitory transmitter. Aspartic acid also appears to be an excitatory transmitter in the spinal cord gray matter. It appears to be associated with interneurons and may oppose the inhibiting action of glycine or GABA-releasing inhibitory interneurons. The formation of these amino acid transmitters from TCA cycle intermediates is illustrated in Fig.
|
NEUROPEPTIDES
 | General Considerations
Over the years, a number of neuropeptides have been identified which play a variety of functional roles in the nervous system. Several have well-known endocrine roles such as ACTH, oxytocin, and vasopressin from the pituitary gland. Also included are the hypothalamic factors which control the release of certain pituitary hormones. These are somatostatin (growth hormone-inhibiting factor), thyrotropin-releasing factor (TRF), and luteinizing hormone-releasing factor (LHRF).
Other neuropeptides appear to function as neurotransmitters. One of these is substance P, found in certain pathways in the brain and in terminal endings of specific primary sensory fibers of spinal nerves. The latter are represented by those fibers which synapse on secondary spinal cord neurons responding most readily to pain. Thus it is hypothesized to operate as a transmitter for painful stimuli from the periphery to the CNS.
Perhaps the most interesting group of neuropeptides are the enkephalins and endorphins. The morphinelike enkephalins have been found in interneurons in the same regions of the spinal cord where substance P is released. and there is evidence to suggest that they inhibit the release of substance P. Thus, enkephalin-containing neurons may work to suppress the transmission of painful information between primary and secondary neurons. Enkephalins probably operate by presynaptically inhibiting the release of substance P from primary neurons, giving them a modulatory role at these synapses.
Enkephalin is also found in several areas of the brain and brainstem, paralleling the distribution of opiate receptors. The highest concentration occurs in the globus pallidus with lesser amounts in the caudate nucleus, hypothalamus, periaqueductal gray matter, and amygdala. The intriguing possibility exists that enkephalins may be naturally occurring analgesics operating as modulating neurotransmitters in various pain-mediating pathways |
|
|
|

Hiç yorum yok:
Yorum Gönder