By the late 1970s, somatosensory evoked potentials (SEPs) became routinely used to intraoperatively assess the functional integrity of the somatosensory system in the spinal cord during surgical correction for scoliosis. The same SEPs data were also routinely extrapolated to assess the functional integrity of the upper motor neuron tracts; however, as data mounted, this approach proved unreliable:
(a) it provided false results when SEPs were found to be present despite postoperative motor deficits;
(b) it provided unreliable (low-quality) or unmonitorable (complete absence) SEPs in patients in whom certain pathologies affected the somatosensory system; and
(c) because dorsal myelotomy often destroyed the dorsal column’s integrity in patients undergoing surgery for intramedullary spinal cord tumors, the ability to monitor SEPs immediately nullifies.
Because of these difficulties, ION was forced to search for more reliable methods to assess the motor system’s functional integrity. Initial attempts to monitor motor tracts in the spinal cord were made and focused on two neurophysiological techniques: spinal-cord-to-spinal- cord recording, and spinal-cord-to-muscle/peripheral-nerve recording.
(a) it provided false results when SEPs were found to be present despite postoperative motor deficits;
(b) it provided unreliable (low-quality) or unmonitorable (complete absence) SEPs in patients in whom certain pathologies affected the somatosensory system; and
(c) because dorsal myelotomy often destroyed the dorsal column’s integrity in patients undergoing surgery for intramedullary spinal cord tumors, the ability to monitor SEPs immediately nullifies.
Because of these difficulties, ION was forced to search for more reliable methods to assess the motor system’s functional integrity. Initial attempts to monitor motor tracts in the spinal cord were made and focused on two neurophysiological techniques: spinal-cord-to-spinal- cord recording, and spinal-cord-to-muscle/peripheral-nerve recording.
| Spinal cord to spinal cord |
This technique operates with nonselective electrical stimulation of the spinal cord and with nonselective recordings of elicited potentials from the spinal cord. It is used to record signals from the spinal cord regardless of the direction of propagation of the action potentials (either ascending, descending, or ortho/antidromic). The type of action potential recorded depends on the position of the stimulating and recording electrodes and the direction of the traveling waves through the spinal cord with regards to the natural direction of the conducting
pathways.
The evoked potentials recorded from the spinal cord using this technique are the electrical sum of activity from multiple pathways. Because of the different conduction properties of the various spinal cord pathways, the recorded potentials can show two distinctive wave morphologies. It has been speculated that one of these waves represents transmission in the dorsal columns (DCs) and the other by the corticospinal tract (CT). Clinical testing on a large number of patients with different and relevant pathologies has not been done to confirm this hypothesis.
This method can evaluate the integrity of ascending and descending, and probably propriospinal pathways, within the spinal cord. However, specific information about the DC or CT cannot be obtained with this method. Critical reports could not confirm the value of the spinal cord to spinal cord technique in monitoring motor pathways during surgery for intramedullary spinal cord tumors.
pathways.
The evoked potentials recorded from the spinal cord using this technique are the electrical sum of activity from multiple pathways. Because of the different conduction properties of the various spinal cord pathways, the recorded potentials can show two distinctive wave morphologies. It has been speculated that one of these waves represents transmission in the dorsal columns (DCs) and the other by the corticospinal tract (CT). Clinical testing on a large number of patients with different and relevant pathologies has not been done to confirm this hypothesis.
This method can evaluate the integrity of ascending and descending, and probably propriospinal pathways, within the spinal cord. However, specific information about the DC or CT cannot be obtained with this method. Critical reports could not confirm the value of the spinal cord to spinal cord technique in monitoring motor pathways during surgery for intramedullary spinal cord tumors.
| Spinal cord to peripheral nerve |
This technique operates with nonselective stimulation of the spinal cord and selective recordings from the peripheral nerves or muscles. Recordings from the muscle and peripheral nerves presume that after electrical stimulation of the spinal cord, α-motoneurons are activated only by the CT tract. Therefore, compound muscle action potentials (CMAPs) in the limb muscles or electrical activity in the peripheral nerves should be generated by CT stimulation. Unfortunately, α-motoneurons can also be activated by any of the multiple descending tracts within the spinal cord after diffuse electrical stimulation of the spinal cord and/or by antidromically activated dorsal columns and their segmental branches that mediate the H reflex. Electrical activity recorded from mixed peripheral nerves is a combination of α-motoneuron discharges initiated by the CT and other descending tracts. Because the sensory component of mixed peripheral nerves is a physical continuation of the dorsal columns, part of the electrical activity recorded from mixed peripheral nerves after stimulation of the spinal cord arises from the antidromically activated dorsal columns that convey traveling waves to the peripheral nerves. Collision studies have challenged the widely accepted presumption that potentials recorded from peripheral nerves in the lower extremities after stimulation of the spinal cord are generated by the CT. Therefore, there is convincing evidence that selective recording of the electrical activity from peripheral nerves elicited by electrical stimulation of the spinal cord does not arise from the CT. Additional evidence concerning the inaccuracy of monitoring the motor pathways through potentials recorded from peripheral nerves is provided by observations of resulting paraplegia in spite of preservation of these potentials.
It is fair to say that both of the techniques described can grossly monitor the functional integrity of multiple pathways inside the spinal cord without being specific for any of them. In other words, these methods can indicate that certain lesions to the spinal cord have occurred, but they lack the ability to provide specific information as to which of the spinal cord pathways has been damaged. This methodology may be useful in orthopedic surgical procedures and other surgeries where lesioning of the nervous tissue within the spinal cord is diffuse in nature and where all pathways are usually affected. An exception to this phenomenon involves vascular lesions of the spinal cord where selective lesioning of the anterolateral columns can occur.
Unfortunately, this nonselective evaluation of multiple pathways is not sufficient during surgery of the spinal cord, during which the DCs can be independently damaged from the anterior and lateral columns. Furthermore, these two techniques cannot evaluate the functional integrity of the CT from the motor cortex to the upper cervical spinal cord. Therefore, supratentorial, brainstem, foramen magnum, and upper cervical spinal cord surgeries cannot be monitored using these techniques. This is also the case in procedures involving the clipping of an intracerebral aneurysm, where the perforating branches for the CT tract in the internal capsula can be selectively damaged while leaving the lemniscal pathways intact. This results in a so-called pure motor hemiplegia (i.e., the patient is postoperatively hemiplegic while the sensory system is intact and SEPs are present). Since it requires the motor cortex to be surgically exposed, Penfield’s technique may not be used for monitoring motor tracts within the spinal cord.
It is fair to say that both of the techniques described can grossly monitor the functional integrity of multiple pathways inside the spinal cord without being specific for any of them. In other words, these methods can indicate that certain lesions to the spinal cord have occurred, but they lack the ability to provide specific information as to which of the spinal cord pathways has been damaged. This methodology may be useful in orthopedic surgical procedures and other surgeries where lesioning of the nervous tissue within the spinal cord is diffuse in nature and where all pathways are usually affected. An exception to this phenomenon involves vascular lesions of the spinal cord where selective lesioning of the anterolateral columns can occur.
Unfortunately, this nonselective evaluation of multiple pathways is not sufficient during surgery of the spinal cord, during which the DCs can be independently damaged from the anterior and lateral columns. Furthermore, these two techniques cannot evaluate the functional integrity of the CT from the motor cortex to the upper cervical spinal cord. Therefore, supratentorial, brainstem, foramen magnum, and upper cervical spinal cord surgeries cannot be monitored using these techniques. This is also the case in procedures involving the clipping of an intracerebral aneurysm, where the perforating branches for the CT tract in the internal capsula can be selectively damaged while leaving the lemniscal pathways intact. This results in a so-called pure motor hemiplegia (i.e., the patient is postoperatively hemiplegic while the sensory system is intact and SEPs are present). Since it requires the motor cortex to be surgically exposed, Penfield’s technique may not be used for monitoring motor tracts within the spinal cord.
| Transcranial Electrical Stimulation |
High-voltage current applied over the skull could penetrate to the brain and activate the motor cortex and the CT. Although they produced discomfort, these methods of transcranial electrical stimulation (TES) became an additional tool used to diagnose upper motoneuron lesions in awake patients. Two methodologies for monitoring the CT intraoperatively are available, the single-pulse stimulation technique and the multipulse stimulation technique.
| Single Pulse Stimulation Technique |
A single-pulse stimulating technique involves a single electrical stimulus applied transcranially or over the exposed motor cortex while the descending volley of the CT is recorded over the spinal cord as a direct wave (D wave).
| Multipulse Stimulation Technique |
A multipulse stimulating technique involves a short train of five to seven electrical stimuli applied transcranially or over the exposed motor cortex while muscle motor-evoked potentials (MEPs) from limb muscles in the form of CMAPs are recorded (Fig.1). (This latter technique differs essentially from the Penfield technique in that it calls for only five to seven stimuli with a stimulating rate of up to 2 Hz. Penfield’s technique calls for continuous stimulation over a period of a few seconds with a frequency of stimulation of 50–60 Hz, and only in the cases when the motor cortex is surgically exposed. Furthermore, at such frequencies and train durations, seizures are easily induced.)
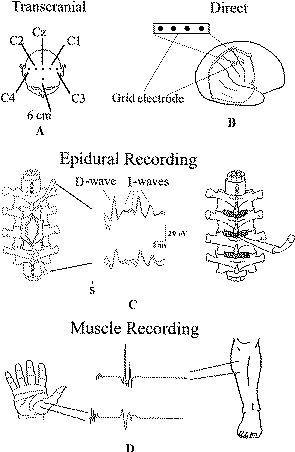 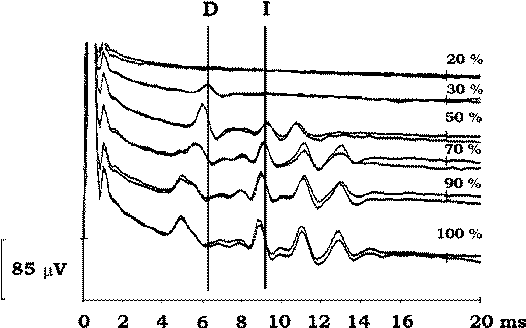 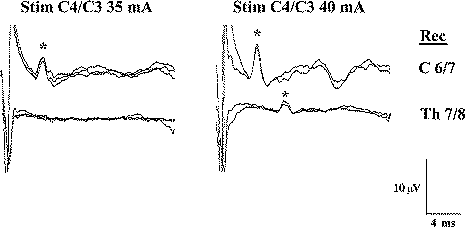 | ||
Figure-1: (A) Schematic illustration of electrode positions for transcranial electrical stimulation of the motor cortex according to the International 10–20 EEG system. The site labeled “6 cm” is 6 cm anterior to CZ. (B) Illustration of grid electrode overlying the motor and sensory cortexes. (C) Schematic diagram of the positions of the catheter electrodes (each with three recording cylinders) placed cranial to the tumor (control electrode) and caudal to the tumor to monitor the descending signal after it passes through the site of surgery (left). In the middle are D and I waves recorded rostral and caudal to the tumor site. On the right is depicted the placement of an epidural electrode through a flavectomy/flavotomy when the spinal cord is not exposed. (D) Recording of muscle motor evoked potentials from the thenar and tibialis anterior muscles after being elicited with multipulse stimuli applied either transcranially or over the exposed motor cortex.
| Figure-2 D and I waves recorded after a single electrical stimulus delivered transcranially (CZ anode/6 cm anterior cathode). When the intensity of the stimulus is increased, electrical current activates the CT deeper within the brain and the latency of the D wave becomes shorter. As current becomes stronger, more I waves are induced (100% corresponds to 750 volts of stimulator output). | Figure-3 Transcranial electrical stimulation over the C4 anode/C3 cathode with recordings of the D wave over the C6–C7 segment (above) and the T7–T8 segment of the spinal cord (below). Stimulus intensity was 35 and 40 mA, respectively. Stronger stimuli elicit the D wave over the thoracic spinal cord, while a weaker stimulus (35 mA) elicits the D wave only over the cervical spinal cord. |
| Scalp Electrode Placement during TES |
The electrode placement on the skull is based on the international 10–20 EEG system. Note that, instead of CZ, the CZ electrode is placed 1 cm behind the typical CZ point. For transcranial stimulation, cork screw–like electrodes are preferable because of their secure placement and low impedance (usually 1 KΩ).
Alternatively, an EEG needle electrode may be used. It is not recommended to use the EEG cup electrodes fixed with collodium since they are impractical and their placement is time-consuming. The only exception is for young children in whom the fontanel still exists. Since the cork screw–like electrodes could penetrate the fontanel during placement, the use of EEG cup electrodes is suggested.
The skull presents a barrier of high impedance to the electrode current applied transcranially; therefore, we cannot completely control the spread of electrical current when it is applied. For this reason, various combinations of electrode montages may need to be explored to obtain an optimal response.
The standard montage is C3/C4 for eliciting MEPs in the upper extremities and C1/C2 for eliciting MEPs in the lower extremities. With sufficient intensity of stimulation at C1/C2, MEPs are preferentially elicited in the right limb muscles while stimulation at C2/C1 elicits MEPs in the left limb muscles.
With stronger electrical stimulation, the current will penetrate the brain more deeply, stimulating the CT at a different depth from the motor cortex (Fig-2). On the basis of measurements of the D wave latency, it has been postulated that there are three favorable points that are susceptible to depolarization of the CT: cortex/subcortex (weak electrical stimulation), internal capsula (moderate electrical stimulation), and brainstem/foramen magnum (strong electrical stimulation). Selectivity of stimulation is possible at the level of the cortex (subcortex). Therefore, only the application of relatively weak electrical stimuli to the cortex is selective, and it activates only a small portion of the CT fibers (e.g., activating only one extremity) or only one CT. It is important to remember that during electrical stimulation of the motor cortex, the anode is preferentially the stimulating electrode. With increasing intensity of the current, the cathode becomes the stimulating electrode as well.
As an example, stimulation with the C3+/C4− will selectively activate muscles of the right arm. When stimulation intensity is increased, the cathode (C4−) becomes the stimulating electrode as well, resulting in the stimulation of the left arm. Finally, when current intensity becomes strong enough to penetrate to the internal capsule more caudally, all four extremity muscles can be activated. For anatomical reasons (deep position of the leg motor area in the interhemispheric fissure), more intense current is usually needed to obtain MEPs in the lower extremities. It is especially difficult to obtain them separately without also activating the upper extremities, but it can be done in certain patients, especially when using the CZ/6 cm in front montage (see Fig-1).
By their anatomical location, recording electrodes in the limb muscles can indicate which fibers of the CT are activated predominantly (left or right, fibers for upper or lower extremities). If one would like to activate left and right CT simultaneously to obtain D wave recordings, weak electrical stimulation should be avoided and a moderate intensity should be used. In Fig-3, it is obvious that weak electrical stimulation activates fibers of the CT for the left upper extremities only. This can result in activation of only one CT while not affecting the other CT. Therefore, the intensity of electrical stimulation for eliciting a D wave should be determined by simultaneous recordings of MEPs from limb muscles (indicating which fibers of the CT have been predominantly activated), or only moderate intensities of electrical current for eliciting D waves should be used. The moderate intensity of electrical current will activate both CTs at the level of the internal capsule. If MEP waves have not been simultaneously recorded with D waves, the following guidelines should be followed: increase the intensity of the stimulation until D waves do not increase in amplitude (Fig-2, the third trace from the top). This is a sign that most of the fast conducting neurons of CT from the left and right CT have been activated.
The neurophysiological mechanism for eliciting MEPs by stimulating the motor cortex in patients under the influence of anesthetics is different from the mechanism in the awake subject. In the latter, electrical current stimulates the body of the motor neuron transynaptically over the chain of vertically oriented excitatory neurons, resulting in I waves (indirect activation of the motoneurons). At the same time, electrical current activates axons of the cortical motoneurons, directly generating D waves. In anesthetized patients, anesthetics block the synapses of the vertically oriented excitatory chains of neurons terminating on the cortical motoneuron’s body. Therefore, only the D wave is generated after electrical stimulation of the motor cortex. Patients with idiopathic scoliosis are an exception. In this group, abundant I waves can be recorded). This is one of the neurogenic markers of the disease present in these patients. Furthermore, it has been shown that a frontally oriented cathode preferentially generates I waves because at this stimulating setting corticocortical projections of vertically oriented interneurons are optimally activated. With the cathode in the lateral position, this is not the case (Fig-4).
Alternatively, an EEG needle electrode may be used. It is not recommended to use the EEG cup electrodes fixed with collodium since they are impractical and their placement is time-consuming. The only exception is for young children in whom the fontanel still exists. Since the cork screw–like electrodes could penetrate the fontanel during placement, the use of EEG cup electrodes is suggested.
The skull presents a barrier of high impedance to the electrode current applied transcranially; therefore, we cannot completely control the spread of electrical current when it is applied. For this reason, various combinations of electrode montages may need to be explored to obtain an optimal response.
The standard montage is C3/C4 for eliciting MEPs in the upper extremities and C1/C2 for eliciting MEPs in the lower extremities. With sufficient intensity of stimulation at C1/C2, MEPs are preferentially elicited in the right limb muscles while stimulation at C2/C1 elicits MEPs in the left limb muscles.
With stronger electrical stimulation, the current will penetrate the brain more deeply, stimulating the CT at a different depth from the motor cortex (Fig-2). On the basis of measurements of the D wave latency, it has been postulated that there are three favorable points that are susceptible to depolarization of the CT: cortex/subcortex (weak electrical stimulation), internal capsula (moderate electrical stimulation), and brainstem/foramen magnum (strong electrical stimulation). Selectivity of stimulation is possible at the level of the cortex (subcortex). Therefore, only the application of relatively weak electrical stimuli to the cortex is selective, and it activates only a small portion of the CT fibers (e.g., activating only one extremity) or only one CT. It is important to remember that during electrical stimulation of the motor cortex, the anode is preferentially the stimulating electrode. With increasing intensity of the current, the cathode becomes the stimulating electrode as well.
As an example, stimulation with the C3+/C4− will selectively activate muscles of the right arm. When stimulation intensity is increased, the cathode (C4−) becomes the stimulating electrode as well, resulting in the stimulation of the left arm. Finally, when current intensity becomes strong enough to penetrate to the internal capsule more caudally, all four extremity muscles can be activated. For anatomical reasons (deep position of the leg motor area in the interhemispheric fissure), more intense current is usually needed to obtain MEPs in the lower extremities. It is especially difficult to obtain them separately without also activating the upper extremities, but it can be done in certain patients, especially when using the CZ/6 cm in front montage (see Fig-1).
By their anatomical location, recording electrodes in the limb muscles can indicate which fibers of the CT are activated predominantly (left or right, fibers for upper or lower extremities). If one would like to activate left and right CT simultaneously to obtain D wave recordings, weak electrical stimulation should be avoided and a moderate intensity should be used. In Fig-3, it is obvious that weak electrical stimulation activates fibers of the CT for the left upper extremities only. This can result in activation of only one CT while not affecting the other CT. Therefore, the intensity of electrical stimulation for eliciting a D wave should be determined by simultaneous recordings of MEPs from limb muscles (indicating which fibers of the CT have been predominantly activated), or only moderate intensities of electrical current for eliciting D waves should be used. The moderate intensity of electrical current will activate both CTs at the level of the internal capsule. If MEP waves have not been simultaneously recorded with D waves, the following guidelines should be followed: increase the intensity of the stimulation until D waves do not increase in amplitude (Fig-2, the third trace from the top). This is a sign that most of the fast conducting neurons of CT from the left and right CT have been activated.
The neurophysiological mechanism for eliciting MEPs by stimulating the motor cortex in patients under the influence of anesthetics is different from the mechanism in the awake subject. In the latter, electrical current stimulates the body of the motor neuron transynaptically over the chain of vertically oriented excitatory neurons, resulting in I waves (indirect activation of the motoneurons). At the same time, electrical current activates axons of the cortical motoneurons, directly generating D waves. In anesthetized patients, anesthetics block the synapses of the vertically oriented excitatory chains of neurons terminating on the cortical motoneuron’s body. Therefore, only the D wave is generated after electrical stimulation of the motor cortex. Patients with idiopathic scoliosis are an exception. In this group, abundant I waves can be recorded). This is one of the neurogenic markers of the disease present in these patients. Furthermore, it has been shown that a frontally oriented cathode preferentially generates I waves because at this stimulating setting corticocortical projections of vertically oriented interneurons are optimally activated. With the cathode in the lateral position, this is not the case (Fig-4).
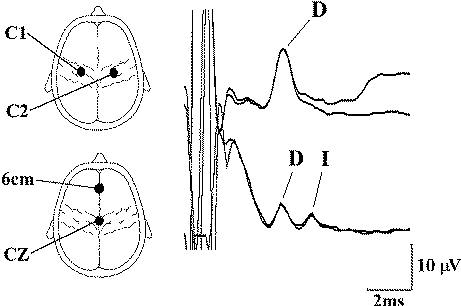  | |
| Figure-4 Upper thoracic epidural recordings of D and I waves in a 14-year-old female during surgery for a low cervical intramedullary tumor. The upper trace was obtained after transcranial electrical stimulation over C1 (anode) and C2 (cathode) using 140 mA stimulus intensity and a stimulus duration of 500 μs. The lower trace was obtained after anodic stimulation at CZ and cathodal stimulation at 6 cm anterior to CZ, using the same stimulus duration but at 200 mA. Note the appearance of the D and I waves with this electrode arrangement. (An upward deflection is negative.) |
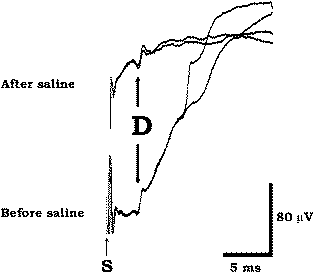
Figure-5 Two traces with a D wave recorded epidurally at the lower cervical spinal cord after percutaneous placement of the epidural electrode in a patient with a brain tumor. High impedance results in a large artifact (lower trace) which has been reduced (upper trace) after injection of saline into the epidural space.
|
| D-wave Recording Technique |
Practically any type of catheter-type electrode designed for electrical stimulation of the spinal cord epidurally can be used for recording D and I waves. The electrode has three platinum-iridium recording cylinders 3 mm in length, 1.3 mm in diameter, and 18 mm apart, with recording surfaces of approximately 12.3 mm2. The electrode is semi-rigid, a property that facilitates its placement either percutaneously or through flavotomy. Furthermore, some electrodes consists of a double lumen with two openings at the tip of the electrode to allow the injection of saline to flush the recording contact surfaces and reduce impedance. This is an important detail in the case of bad electrode contact if the electrode is placed percutaneously in the epidural space (where it can face a high impedance). Once the electrode is in place, it is very difficult to reposition it.
Thus an injection of saline through the outer lumen is a method of rectifying the high-impedance problem (Fig-5). When the electrode is placed after laminectomy, problems with impedance and positioning of the electrode are easier to solve because the surgeons are able to reposition the lead. Most epidural electrodes are disposable. If one uses a nondisposable type, extreme care should be taken to ensure that the electrode is clean before sterilization and thus has improved electrical properties. To clean the electrode, you can immerse the electrode tip in saline and pass a 9 V DC current (regardless of polarity) through it until a bubble of gas cleans the contact surface for a period of a few minutes, or you can use an ultrasound cleaner by submersing the electrode in the cleaner for 5 minutes. Both techniques will remove any film or biological material remaining on the electrode from the contact surfaces and will decrease their impedance. This maneuver will diminish the stimulus artifact, which usually appears when contact surfaces have high impedance. Because of the short latency of the D wave, a large stimulus artifact in an uncleaned electrode can pose an insurmountable obstacle for D wave recording.
Thus an injection of saline through the outer lumen is a method of rectifying the high-impedance problem (Fig-5). When the electrode is placed after laminectomy, problems with impedance and positioning of the electrode are easier to solve because the surgeons are able to reposition the lead. Most epidural electrodes are disposable. If one uses a nondisposable type, extreme care should be taken to ensure that the electrode is clean before sterilization and thus has improved electrical properties. To clean the electrode, you can immerse the electrode tip in saline and pass a 9 V DC current (regardless of polarity) through it until a bubble of gas cleans the contact surface for a period of a few minutes, or you can use an ultrasound cleaner by submersing the electrode in the cleaner for 5 minutes. Both techniques will remove any film or biological material remaining on the electrode from the contact surfaces and will decrease their impedance. This maneuver will diminish the stimulus artifact, which usually appears when contact surfaces have high impedance. Because of the short latency of the D wave, a large stimulus artifact in an uncleaned electrode can pose an insurmountable obstacle for D wave recording.
This procedure is used usually to monitor the CT during brainstem and supratentorial surgeries where there is high risk of potential damage. Today, because of the increasing popularity of MEPs monitoring during procedures involving the spinal cord and brainstem, the demand (indications) for percutaneous placement of this type of electrode has diminished. A 14-gauge, thin-wall Touhy needle is used for introducing the electrode into the epidural space percutaneously. Following percutaneous electrode placement, care must be taken not to withdraw the electrode while the Touhy needle is in place. Otherwise, the sharp edge of the needle could shred the wall of the electrode. The optimal position for penetrating the epidural space with the Touhy needle is the upper thoracic (T1–T2) epidural space. With the needle in this region, the catheter electrode can be gently pushed up to the level of the lower cervical spinal cord. With this electrode placement we can monitor the CT for both the upper and lower extremities by recording D waves after selective stimulation of the motor cortex. Appropriate electrode placement can be confirmed either by x-ray or by recording epidural SEPs from the same electrode after stimulation of the median or ulnar nerves.
Minimal complications from the placement of the electrode occurs (e.g., bleeding, infection, or puncture of the spinal cord). This method requires skills that the anesthesiologist practiced in the epidural injection of anesthetics would typically have.
Minimal complications from the placement of the electrode occurs (e.g., bleeding, infection, or puncture of the spinal cord). This method requires skills that the anesthesiologist practiced in the epidural injection of anesthetics would typically have.
This technique is regularly used for all procedures that require CT monitoring when a laminectomy is performed. These procedures include surgery for the removal of spinal cord tumors and different surgical interventions on the spinal cord. The surgeon places two catheter electrodes in the epi- or subdural space at the rostral and caudal edge of the laminectomy. The rostral electrode is the control electrode for nonsurgically induced changes in the D wave, while the caudal one monitors the surgically induced changes to the CT (see Fig-1).
Massive dural adhesions, usually from previous surgery or after spinal cord radiation, can prevent the placement of the catheter electrode. Also, placement below the T10 bony level cannot record a D wave of sufficient amplitude because of lack of sufficient CT fibers. The control (rostral) electrode cannot be placed in cases of high cervical spinal cord pathology because of the lack of space. The amplitude of the D wave recorded over the cervical spinal cord could be 60 μV or more, while over thoracic segments it may be only 10 μV. With a stimulating rate of 2 Hz, it takes two to four averaged responses to get a reliable D wave. This results in an update every second. Unfortunately, the maximal stimulating rate from commercially available TES stimulators is 1 stimulus per second.
In surgical procedures in which the spine is exposed but a laminectomy is not performed (e.g., surgical corrections of scoliosis or dorsal approach to spine stabilization), the catheter electrode may be inserted through a flavotomy/flavectomy.
Massive dural adhesions, usually from previous surgery or after spinal cord radiation, can prevent the placement of the catheter electrode. Also, placement below the T10 bony level cannot record a D wave of sufficient amplitude because of lack of sufficient CT fibers. The control (rostral) electrode cannot be placed in cases of high cervical spinal cord pathology because of the lack of space. The amplitude of the D wave recorded over the cervical spinal cord could be 60 μV or more, while over thoracic segments it may be only 10 μV. With a stimulating rate of 2 Hz, it takes two to four averaged responses to get a reliable D wave. This results in an update every second. Unfortunately, the maximal stimulating rate from commercially available TES stimulators is 1 stimulus per second.
In surgical procedures in which the spine is exposed but a laminectomy is not performed (e.g., surgical corrections of scoliosis or dorsal approach to spine stabilization), the catheter electrode may be inserted through a flavotomy/flavectomy.
| FACTORS INFLUENCING D AND I WAVE RECORDINGS |
D waves represent a neurogram of the CT which is not significantly influenced by nonsurgically induced factors. Stimulation of the CT takes place intracranially distal to the cortical motoneuron body, while recording is done caudal to the surgical site but above the synapses of the CT at the α-motoneuron. Since no synapses are involved between the stimulating site and the recording site, the D wave is very stable and reliable. Therefore, we consider D wave recordings to be the “gold standard” for measuring the functional integrity of the CT.
Still, there exists a few nonsurgically induced changes that will affect the D wave. Being able to correctly recognize them is essential to giving the surgeon appropriate information. If the exposed spinal cord is cooled, either by cold irrigation with saline or low operating room temperature, the latency of the D wave will be temporarily prolonged (Fig-6). Sometimes during stimulation, even with a single stimulus, the epidural electrode can pick up the paraspinal muscle artifact. This would affect the I wave, but not the D wave, parameters (see Fig-7). If this phenomenon occurs, it is more frequent during cervical than thoracolumbar catheter placement.
Volatile anesthetics mostly, do not change the parameters of the D wave by influence on the membrane properties of the CT. To demonstrate this, as isoflurane concentration increases (e.g., >2%), the latency of the D wave gets prolonged while the amplitude diminishes (see Fig-8). However, this can be easily corrected by increasing the intensity of the current. Therefore, the mechanism by which isoflurane influences the parameters of the D wave is vasodilatation of the cortical blood vessels. Because of the vasodilatation, current between the stimulating electrodes shunts and activates the CT more superficially, resulting in longer latencies of the D wave. The smaller amplitude of the D wave results from fewer fibers of the CT being activated if current flows superficially (Fig-9). A prolongation of the latency and a diminished amplitude of the D wave occur only if the CT is activated transcranially. In contrast, this phenomenon is not present when the motor cortex is stimulated directly through a grid electrode with a short distance between the electrodes. All of the above observations provide evidence that changes in the D wave are due to mechanisms other than influence on the CT axon membranes.
Still, there exists a few nonsurgically induced changes that will affect the D wave. Being able to correctly recognize them is essential to giving the surgeon appropriate information. If the exposed spinal cord is cooled, either by cold irrigation with saline or low operating room temperature, the latency of the D wave will be temporarily prolonged (Fig-6). Sometimes during stimulation, even with a single stimulus, the epidural electrode can pick up the paraspinal muscle artifact. This would affect the I wave, but not the D wave, parameters (see Fig-7). If this phenomenon occurs, it is more frequent during cervical than thoracolumbar catheter placement.
Volatile anesthetics mostly, do not change the parameters of the D wave by influence on the membrane properties of the CT. To demonstrate this, as isoflurane concentration increases (e.g., >2%), the latency of the D wave gets prolonged while the amplitude diminishes (see Fig-8). However, this can be easily corrected by increasing the intensity of the current. Therefore, the mechanism by which isoflurane influences the parameters of the D wave is vasodilatation of the cortical blood vessels. Because of the vasodilatation, current between the stimulating electrodes shunts and activates the CT more superficially, resulting in longer latencies of the D wave. The smaller amplitude of the D wave results from fewer fibers of the CT being activated if current flows superficially (Fig-9). A prolongation of the latency and a diminished amplitude of the D wave occur only if the CT is activated transcranially. In contrast, this phenomenon is not present when the motor cortex is stimulated directly through a grid electrode with a short distance between the electrodes. All of the above observations provide evidence that changes in the D wave are due to mechanisms other than influence on the CT axon membranes.
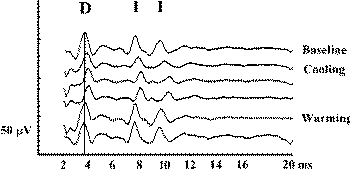 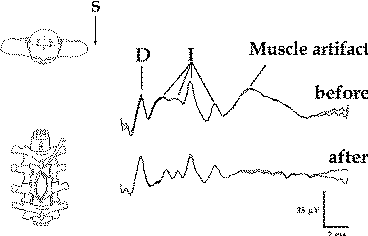 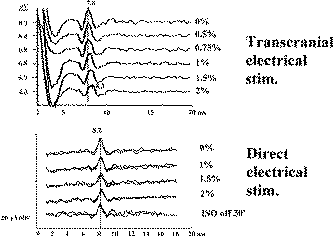 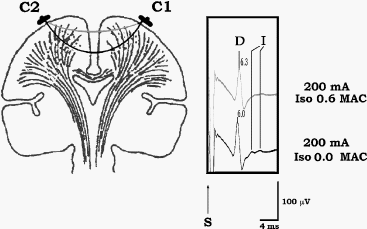 | |||
| Figure-6 D waves recorded over the lower cervical spinal cord in a patient with an upper cervical intramedullary spinal cord tumor, after stimulation with CZ anode/6 cm anterior cathode. Temporary cooling of the exposed spinal cord results in delayed latency of the D and I waves. After warming of the spinal cord, the latency of the D and I waves returned to the previous values. | Figure-7 Epidurally recorded D and I waves over the cervical spinal cord showing a muscle artifact. After administration of the muscle relaxant, the muscle artifact disappears. The muscle artifact affects the I wave, but not the D wave, recordings. S = beginning of transcranially applied stimulus. | Figure-8 Transcranial electrical stimulation (CZ anode/6 cm anterior cathode) and direct electrical stimulation of the exposed motor leg area with recording of the D wave over the lower thoracic spinal cord in two different patients. Identical concentrations of isoflurane showed a prominent effect on the amplitude and latency of the D wave (50% decrement of amplitude and 0.5 ms prolonged latency after end tidal concentration of 2% isoflurane). This effect is only evident when transcranial electrical stimulation is used. A minimal effect of isoflurane on D wave parameters was observed when electrical stimulation was applied to the exposed cortex. | Figure-9 To the left, current flow is represented schematically before (white line) and after (grey line) administration of isoflurane. Because of the vasodilatatory effects of isoflurane on the cortical blood vessels, the current between the two stimulating electrodes is shunted, flowing through the brain more superficially. This results in a prolonged latency and smaller amplitude of the D wave when compared to a D wave elicited with the same intensity of current without isoflurane (6.0 ms vs. 6.3 ms, respectively; to the right). At the same time, the disappearance of the I wave can be observed under the influence of isoflurane. |
| FACTORS LEADING TO THE DESYNCHRONIZATION OF THE D WAVE |
In certain patients with spinal cord tumors (usually involving a few segments) the D wave is not recordable at the beginning of surgery. At the same time, muscle MEPs are recordable, even in patients that may not necessarily have a major motor deficit (Fig-10). The temporal summation of the desynchronized D waves occurs at the segmental level. The same phenomenon is present in patients who undergo radiation of the spinal cord. This is a result of a desynchronization in conduction of the CT axon. In other words, fast fibers of the CT conduct D waves with different speeds over the site of the lesion or irradiation. Therefore, desynchronized D waves cannot be easily demonstrated caudal to the lesion site with the present methodology. There are different grades of desynchronization, which will be seen as low-amplitude and widebase D waves (Fig-10A). A higher degree of desynchronization is represented by a nonrecordable D wave (Fig-10B).
Patients who do not have a recordable D wave at the beginning of surgery are challenging for the monitoring team because they represent a high-risk group of patients for injury to the CT. With the present methodology, you can only monitor them by recording MEPs from limb muscles. Because of the possibility that transient paraplegia may occur, this is not an ideal monitoring tool. When muscle MEPs disappear during surgery in the patients who do not have a recordable D wave at baseline, it is not possible to distinguish transient from permanent motor deficit intraoperatively.
Patients who do not have a recordable D wave at the beginning of surgery are challenging for the monitoring team because they represent a high-risk group of patients for injury to the CT. With the present methodology, you can only monitor them by recording MEPs from limb muscles. Because of the possibility that transient paraplegia may occur, this is not an ideal monitoring tool. When muscle MEPs disappear during surgery in the patients who do not have a recordable D wave at baseline, it is not possible to distinguish transient from permanent motor deficit intraoperatively.
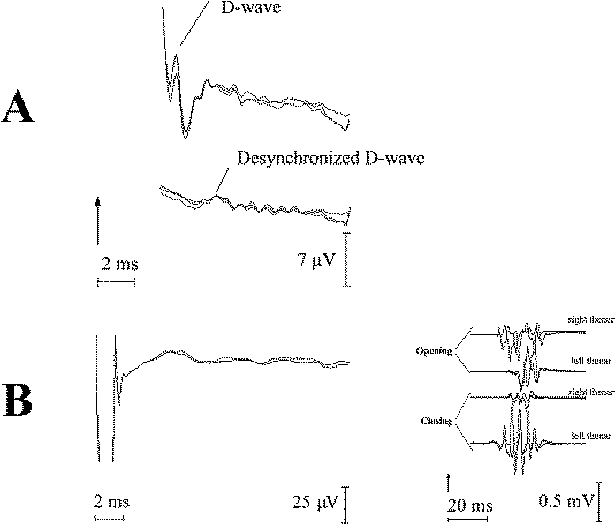 |
| Figure-10 (A) Recording of a D wave cranially (upper trace) and caudally (lower trace) to the intramedullary spinal cord tumor. Note the well-synchronized D wave cranially, in contrast to the desynchronized D wave caudal to the tumor. (B) Very small epidurally recorded MEPs caudal to a high cervical intramedullary tumor (due to extreme desynchronization), despite large muscle MEPs recorded from a small hand muscle elicited after a short train of six stimuli were present (to the right). |
| SELECTION OF OPTIMAL MUSCLES IN UPPER AND LOWER EXTREMITIES FOR MEP RECORDINGS |
The selection of appropriate muscles to record from is an important issue in the monitoring of MEPs. In certain patients who have deep paresis, not choosing the optimal muscles can result in “nonmonitorable” patients. The small hand muscle (e.g., abductor pollicis brevis, or APB) is one of the optimal muscles to monitor the CT for the upper extremities. It has been shown that a good alternative is the long forearm flexors, or even the forearm extensors. The spinal motoneurons for these muscle groups have rich CT innervation and are therefore suitable for monitoring the functional integrity of the CT. This is not the case with the proximal muscle of the arm or of the shoulder (biceps, triceps, or deltoid muscles).
For the lower extremities, abductor hallucis brevis (AHB) is the optimal muscle because of its dominant CT innervation. In animal experiments, it has been shown that after CT stimulation the highest amplitude of the excitatory postsynaptic potential (EPSP) has been found in the α-motoneuron pools for the lower extremities in the small and long flexors of the foot. An alternative to this muscle is the tibialis anterior muscle (TA). The standard electrode montage for recording MEPs in the upper and lower extremities are the AHB and TA for the lower extremities and the ABP for the upper extremities.
For the lower extremities, abductor hallucis brevis (AHB) is the optimal muscle because of its dominant CT innervation. In animal experiments, it has been shown that after CT stimulation the highest amplitude of the excitatory postsynaptic potential (EPSP) has been found in the α-motoneuron pools for the lower extremities in the small and long flexors of the foot. An alternative to this muscle is the tibialis anterior muscle (TA). The standard electrode montage for recording MEPs in the upper and lower extremities are the AHB and TA for the lower extremities and the ABP for the upper extremities.
| MECHANISMS FOR ELICITING MEPS USING A MULTIPULSE STIMULATION TECHNIQUE |
Understanding the mechanism involved in the generation of MEPs is essential for describing their appropriate use, explaining their behavior, understanding their value, and knowing their limits during the monitoring of the CT. Generation of MEPs is more complex in nature than the generation of the D and I waves. Therefore, their interpretation, especially during anesthesia, is rather complex. Generation of MEPs and their propagation to the end organ (muscle) depends on (a) the excitability of the motor cortex and the CT tract, (b) the conductivity of CT axons, (c) the excitability level of α-motoneuron pools, (d) the role played by the supportive system of the spinal cord (helping to increase the excitability of α-motoneurons), and (e) the integrity of motor nerves, the motor endplates and muscles.
There is a frequency limit for the transmission of descending volleys through the CT axons to the α-motoneurons. This limit can be easily tested by applying two identical electrical stimuli transcranially with different interstimulus intervals (ISIs). This test can show the recovery time of the second D wave response.
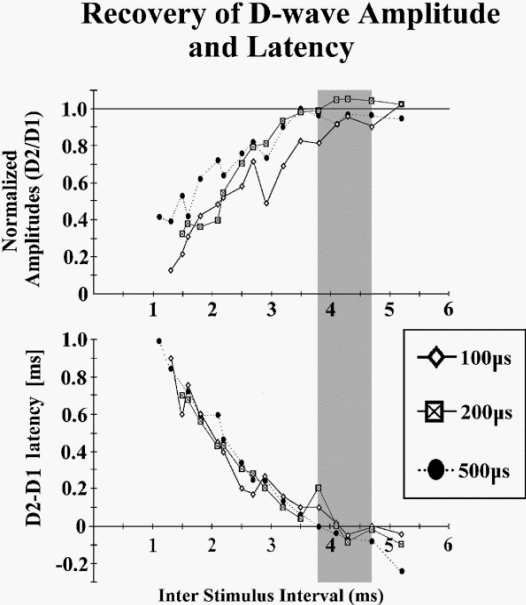 | 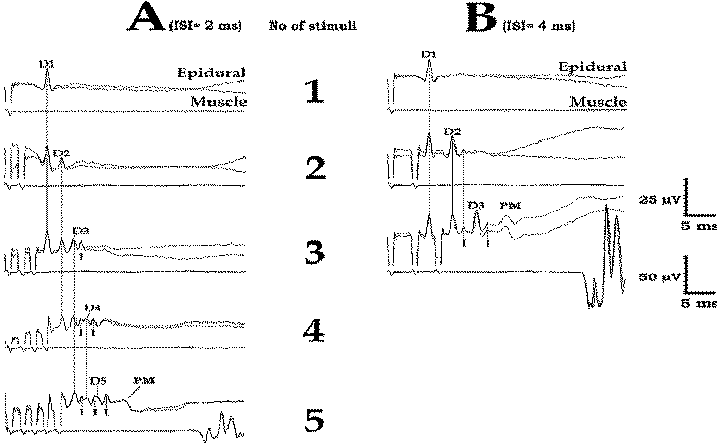 |
Figure-11 Two diagrams showing the relationship between interstimulus interval (ISI), duration of stimuli, and recovery of the amplitude and latency of the conditioning D wave. Two identical stimuli have been applied transcranially with different ISIs. Amplitude and latency of the second D wave (D2) were compared to those of the first one (D1). Note that earlier and complete recovery of the amplitude and latency of the second D wave occurs with a stimulus duration of 500 μs and an ISI of around 4 ms.
| Figure-12 Relationship between MEPs recorded epidurally and from muscle. (A) Train of five stimuli are needed with an ISI of 2 ms in order to elicit muscle MEPs in tibialis anterior muscle (A5). (B) With an ISI of 4 ms, only three stimuli are needed to elicit muscle MEPs in the tibialis anterior muscle (B3). D = D wave; I = I wave; PM = paraspinal muscle artifact. |
Using this paradigm (conditioning and test stimuli), a D wave recovery curve can be plotted relative to the amplitude and latency of the second D wave (Fig.2-11). The optimal ISI for complete recovery of the second D wave amplitude and latency is around 4 ms, using a moderate stimulus intensity with a duration of 500 μs. Because the α-motoneuron is optimally bombarded when the train of equal stimuli elicits D waves of equal amplitudes, the optimal ISI for muscle activation is expected to be 4 ms. Fig-12 indicates that with an ISI of 4 ms, three stimuli are sufficient to elicit MEPs because of the complete recovery of each consecutive D wave (Fig-12 B3). Comparatively, using the identical stimulus intensity but decreasing the ISI to 2 ms, five stimuli are needed to elicit MEPs, which are of even smaller amplitude, because of incomplete recovery of the amplitude of each consecutive D wave (Fig-12 A5). This rule applies only if a single stimulus elicits a single D wave.
We have been shown that three stimuli applied transcranially over the motor cortex can elicit more than three descending volleys in lightly anesthetized patients. In Fig-12 A3, it is clearly visible that three stimuli generate four descending volleys (D1, D2, D3, and an additional I wave). Facilitation of previously nonexisting I waves (after a single stimulus, Fig-12 A1) is one of the important factors underlying the potency of the multipulse stimulating technique for eliciting MEPs in lightly anesthetized patients. Furthermore, it has been shown that because of the lack of synchronicity of I waves, their recorded amplitude is only one third of their actual amplitude. Certainly, if the patient is deeply anesthetized, the cortical synapses where the I wave was facilitated are completely blocked, so this phenomenon does not occur.
To allow for the complete recovery of the D wave, the ISI in the multipulse train should be 4 ms. In situations where a single stimulus generates more than a single D wave, the optimal ISI should be set long enough to allow the entire set of D and I waves to recover, and in turn, to allow the next set of D and I waves to fully develop. Therefore, the second stimulus can generate the same pattern of D and I waves (Fig-13). Otherwise, the second set of D and I waves could fall into the CT axon refractory period resulting from the first set. This is the case in Fig-13, where a single stimulus generates a single D wave and multiple I waves (A). In this case only two stimuli, 8 ms apart, were necessary to generate the maximum amplitude of muscle MEPs (Fig-13D).
If the ISI is shorter (e.g., 4.1 ms in Fig-13B), partial cancellation of the D and I waves elicited by a second stimulus will occur. Consequently, the total number of D and I waves will be insufficient to bring an α-motoneuron to the firing level and MEPs will not be generated. This mechanism could be important in the lightly anesthetized patient as well as in patients with idiopathic scoliosis where a single stimulus generates multiple I waves (see Fig-2).
If the ISI is shorter (e.g., 4.1 ms in Fig-13B), partial cancellation of the D and I waves elicited by a second stimulus will occur. Consequently, the total number of D and I waves will be insufficient to bring an α-motoneuron to the firing level and MEPs will not be generated. This mechanism could be important in the lightly anesthetized patient as well as in patients with idiopathic scoliosis where a single stimulus generates multiple I waves (see Fig-2).
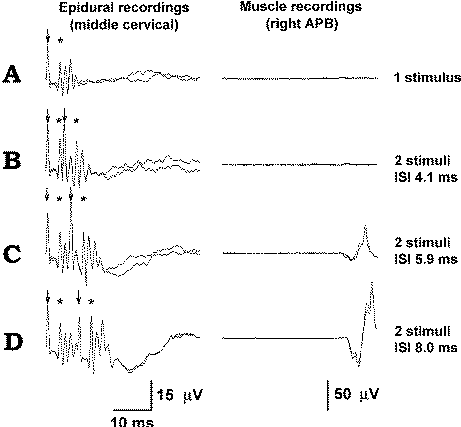 |
Figure-13 (A) In this patient, a single stimulus delivered over the exposed motor hand area elicits a single D wave and multiple I waves. The ISI should be long enough to prevent the second set of D and I waves, elicited by a second stimulus, from falling into the CT axon refractory period resulting from the previous waves (as is the case in trace B). When the ISI is 5.9 ms (C) and 8.0 ms (D), this will not occur, resulting in a sufficient numbers of D and I waves to elicit MEPs (trace D). The stimulus is marked by an arrow and the D wave by an asterisk.
|
| Generation of Muscle MEPs Depends on Two Systems: The CT and the Supportive System of the Spinal Cord |
Descending activity from the CT axons alone is not sufficient to generate muscle MEPs in anesthetized patients. The other system(s) should be activated as well. Three examples support this statement:
A. If the multipulse technique (in a non-deeply anesthetized patient) with a repetition rate of 1 or 2 trains per second is performed, each consecutive response recorded from muscle will have an increasing amplitude. In cases where the intensity of stimuli is just slightly above the threshold, the first few trains will not generate muscle MEPs at all. At the same time, the D wave amplitudes remain the same (Fig-14).
B. In the patients with intramedullary spinal cord tumors presented in Fig.15, recording of the D waves from the left and right CT generates symmetrical D waves cranially and caudally to the tumor site. Yet muscle MEPs are significantly smaller over the right TA muscle where the patient has clinical weakness. The presumption is that the current required to elicit MEPs from muscles on one side of the body is activating only one CT. Therefore, the D wave, recorded from the spinal cord using this same intensity, must predominantly belong to one CT.
C. During surgery for intramedullary spinal cord tumors, muscle MEPs can completely disappear with no significant changes in the amplitude of the D wave (see further transient paraplegia, Fig-16).
These three examples provide convincing evidence that the generation of MEPs involves more than just the CT system.
A. If the multipulse technique (in a non-deeply anesthetized patient) with a repetition rate of 1 or 2 trains per second is performed, each consecutive response recorded from muscle will have an increasing amplitude. In cases where the intensity of stimuli is just slightly above the threshold, the first few trains will not generate muscle MEPs at all. At the same time, the D wave amplitudes remain the same (Fig-14).
B. In the patients with intramedullary spinal cord tumors presented in Fig.15, recording of the D waves from the left and right CT generates symmetrical D waves cranially and caudally to the tumor site. Yet muscle MEPs are significantly smaller over the right TA muscle where the patient has clinical weakness. The presumption is that the current required to elicit MEPs from muscles on one side of the body is activating only one CT. Therefore, the D wave, recorded from the spinal cord using this same intensity, must predominantly belong to one CT.
C. During surgery for intramedullary spinal cord tumors, muscle MEPs can completely disappear with no significant changes in the amplitude of the D wave (see further transient paraplegia, Fig-16).
These three examples provide convincing evidence that the generation of MEPs involves more than just the CT system.
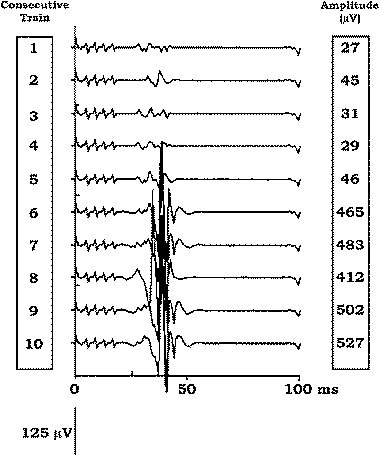 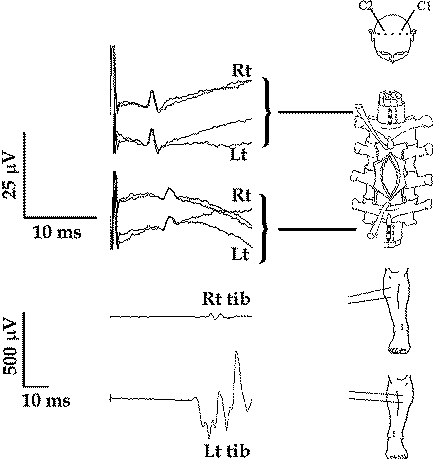 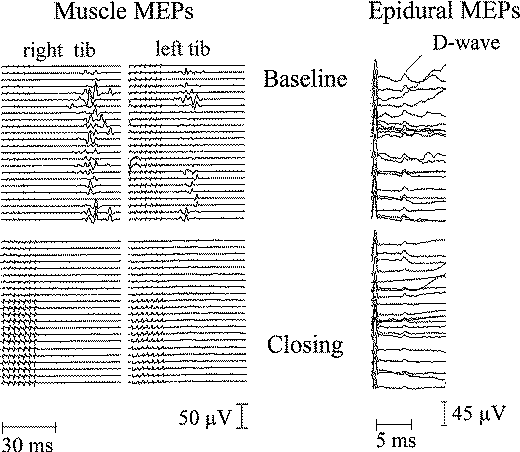 | ||
Figure-14 Recordings of 10 consecutive muscle MEPs from the right abductor hallucis brevis muscle (after delivering 10 trains consisting of five stimuli, pulse width of 100 μs, intensity of 288 mA, stimulus rate of 1 Hz) over C3 anode/C4 cathode in a 60-year-old patient undergoing anterior cervical spine decompression and stabilization. Note that after the fifth train the amplitude of the muscle MEPs increases 10-fold, showing a tendency to further increase its amplitude.
|
Figure-15 Simultaneous recording of the D wave from the right and left CT, cranial and caudal to a midthoracic intramedullary spinal cord tumor (upper), showing a symmetrical amplitude of the D wave. At the same time, muscle MEPs showed significantly smaller amplitude over the right TA muscle when compared to the left, correlating with the patient’s weakness in the right leg. This recording indicates involvement of pathways other than the CT in the generation of the MEPs.
|
Figure-16 Muscle MEPs recorded from right and left TA muscle (left) and D wave recorded epidurally over the lower cervical spinal cord (right). During surgery, muscle MEPs completely disappeared while the D wave decreased in amplitude (less than 50%), resulting in transient paraplegia for this patient during surgery for an intramedullary spinal cord tumor. The patient recovered completely within a week.
|
| SURGICALLY INDUCED TRANSIENT PARAPLEGIA |
During surgery for intramedullary spinal cord tumors in the thoracic region, MEPs in the TA muscles will frequently disappear while the D wave remains unaffected. All patients demonstrating this finding during surgery wake up paraplegic (or monoplegic if the TA MEPs disappear in one leg). In patients in whom was observed this phenomenon, motor strength is typically recover in a few hours to a few days following surgery. No permanent motor deficits have been observed (Fig-16). With almost all cases of transient paraplegia, the first changes are seen in the MEPs and not in the parameters of the D wave. This gives the surgeon a warning sign and a window of time to plan to end the tumor removal. This is a critical point for intraoperative planning of the extent of tumor removal. If changes in the MEPs do not appear, tumor removal can proceed until a gross total resection is accomplished without the patients having permanent motor deficits postoperatively.
Taking into account the previous evidence that the generation of muscle MEPs involves more than just the CT, activation of the CT and other descending systems within the spinal cord is necessary. The propriospinal (diffuse) system of the spinal cord is activated by CT axons that are linked via synaptic connections to the propriospinal system within the spinal cord. In the case of surgically induced transient paraplegia, this system is temporarily compromised by selective surgery while the CT is left intact. After the patient wakes up, other descending systems compensate for the lack of propriospinal tonic influence on α-motoneurons. This results in the fast recovery of these patients. This suggested mechanism is speculative but from a prognostic and pragmatic point of view is critical because it correlates extremely well with clinical outcome. Comparatively, if the CT tract is damaged during surgery (complete loss of D wave or decrement of the amplitude compared with the baseline of more than 50%), a permanent motor deficit isexpected.
Combining the information about the D wave and about the muscle MEPs during surgery for intramedullary spinal cord tumors makes this surgery safer, changes the intraoperative strategy, and significantly diminishes the occurrence of postoperative deficits.
Combining the information about the D wave and about the muscle MEPs during surgery for intramedullary spinal cord tumors makes this surgery safer, changes the intraoperative strategy, and significantly diminishes the occurrence of postoperative deficits.
| Summary |
Historically, intraoperative neurophysiology has progressed by means of trial and error. Unfortunately, this has resulted in a number of different opinions as to its utility in documenting and preventing surgically induced neurological injury. In spite of this, the methodology for monitoring the functional integrity of the CT has progressed over the last 20 years into a reliable, fast, and relatively simple tool that is easily utilized intraoperatively. The development of such a solid methodology has given us reliable and specific data that highly correlate with neurological outcome postoperatively. This correlation and the published surgical outcome data demonstrate the merits of these techniques.
Further developments in intraoperative neurophysiology should be directed toward developing a methodology for the functional mapping of the nervous tissue in the exposed brain, brainstem, and spinal cord during surgery.
Further developments in intraoperative neurophysiology should be directed toward developing a methodology for the functional mapping of the nervous tissue in the exposed brain, brainstem, and spinal cord during surgery.
Hiç yorum yok:
Yorum Gönder