Electroencephalography is the technique by which the electrical activity generated by the brain is amplified and displayed, resulting in an electroencephalogram (EEG). This method enables one to assess brain function noninvasively over a given period. Although many abnormalities on the EEG are nonspecific, several clinical presentations have associated EEG findings that are diagnostic of a specific condition or lesion in the central nervous system. Before the advent of modern neuroimaging, the EEG was one of the most important noninvasive diagnostic tools available to the neurologist and neurosurgeon. It provided information on cerebral function when anatomic detail could not be accurately obtained. Current neuroimaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) of the brain now yield excellent neuroanatomic detail. Despite these advances, the EEG remains a valuable tool in the clinical evaluation of many disorders of the central nervous system, as it is readily available and safe and provides information on brain function that is still unique.
When an EEG is requested, it is important that the referring physician state the clinical question that is to be answered by the EEG. Common reasons for obtaining an EEG include a history of a clinical seizure and the need to rule out epileptiform activity; acute encephalopathy or coma of undetermined etiology; or a prolonged seizure with the need to rule out ongoing electrographic seizure activity (i.e., status epilepticus). When the EEG is completed, the findings are summarized in a report using accepted EEG terminology, with the most significant findings listed first. The EEG is also interpreted in the context of the clinical presentation and question, thus providing the clinician with a clinical correlation to the findings noted in the EEG.
| History |
The early evaluations of the central nervous system by physiologists in the late 1700s and early 1800s consisted of stimulating the brain electrically rather than measuring the electrical currents it generates. Not until the latter half of the nineteenth century did the British physiologist Richard Caton describe the electrical activity of the brain in experimental animals. Caton obtained cortical EEG recordings, and he also noted that' 'feeble currents of varying direction pass through the multiplier when the electrodes are placed on two points of the external surface of the skull. Early in the twentieth century, the Russian physiologist V.V. PravdichNeminsky used the term "electrocerebrogram," and he defined the predominant frequency bands of the cerebral electrical activity in animals, labeling them alpha and beta. In 1929 Hans Berger published the initial findings on the EEG in humans, calling it the "Elektrenkephalogramm", from which electroencephalogram has been derived. Previous investigators had noted the reactivity of the EEG in animals to peripheral somatic electrical stimulation. Berger showed that the human EEG is reactive to opening and closing of the eyes: such potential changes from the occipital region were later termed the Berger, or alpha, rhythm.
In 1934, Berger's findings were confirmed by Adrian and Matthews. The application of EEG in a neuropathologic condition was initially described by Walter when he demonstrated focal EEG slowing in patients with brain tumors, which he called delta waves. During the subsequent two decades, clinical investigators evaluated the use of the EEG in normal and neuropathologic conditions. Over the past six decades. standards have also been developed for the application and nomenclature of electrode placement and montage representation. The clinical significance of most EEG patterns has been well described. Advances in electronics and computers have been applied to electroencephalography, providing improved definition of both cerebral and extracerebral activity (such as artifacts). The EEG now is "paperless," with a digitized EEG displayed in real time on a video monitor. Frequency spectral analysis (brain mapping) is being actively investigated, and it proved to be an additional tool in the evaluation of brain function.
| Technical Aspects |
The electrical activity of the brain has an amplitude in the microvolt range, typically ranging from 10 to 150 µV. In a routine EEG, the brain's electrical activity is measured at the scalp using a surface electrode. The electrical signal is then conducted by wire to the EEG machine, where it is amplified, filtered, and displayed. This process is briefly summarized below.
| Electrodes |
Since the discovery of the EEG. several types of electrodes have been used. Subdermal needle electrodes were the first to be applied. However, owing to the variability of impedance and the potential for morbidity and transmission of infectious disease, this type of electrode is no longer routinely used. The most common electrode currently in use is a gold-plated disc 10 mm in diameter. Twenty-one electrode sites on the scalp are defined according to the International 10-20 System, which is based on skull landmarks (inion, nasion and left and right preauricular points) whose distances are then subdivided in a specified manner. A typical interelectrode distance is 6 cm. Scalp electrodes are identified by a letter and number (Fig-1). Most of the letters specify an approximate brain region. as follows: Fp: frontopolar; F: frontal; C: central; T: temporal; P: parietal; and O: occipital. The ear electrode is denoted by the letter A. Electrodes with odd numbers are on the left side of the head (Fp1, F3, C3, P3, O1, F7, T3, T5, and A1), and electrodes with even numbers are on the right side (Fp2, F4, C4, P4, 02, F8, T4, T6, and A2). Midline electrodes are designated by the letter "z" (Fpz, Fz, Cz, Pz, and Oz).
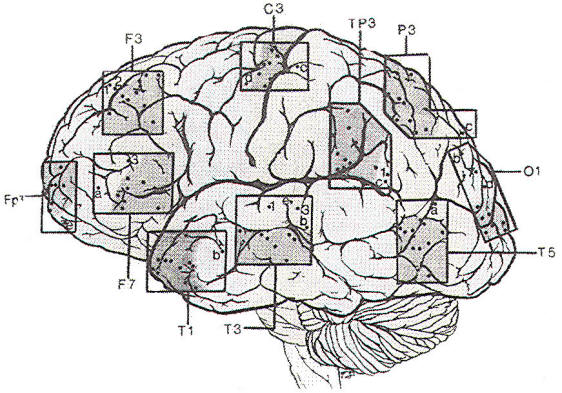 | 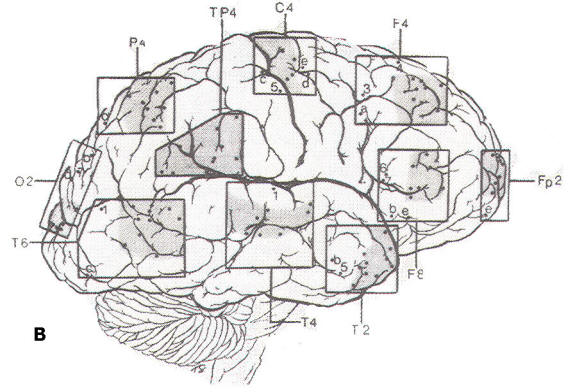 |
| Fig-1: | |
After marking the scalp according to the International 10-20 System, the technologist prepares each site by using a mild abrasive to lower and equalize the scalp impedance. An electrode is placed at each site using either a conductive paste or a collodionsoaked gauze patch through which conductive gel is injected into the disc. Properly prepared electrodes have impedances between 1000 and 5000 Ω.
Scalp electrodes provide adequate measurement of the cerebral electrical activity arising from the superior and lateral aspects of the brain. The anterolateral temporal lobe can be sampled by using a pair of "true" temporal electrodes (T1 and T2), in addition to the 10-20 System electrodes. However, the midline and basal aspects of the brain cannot be sampled well by electrodes on the scalp. In the past the nasopharyngeal electrode was used in an attempt to measure the electrocerebral activity of the anteromesial aspect of the temporal lobe. It consisted of a silver rod that was advanced through the naris until it came in contact with the posterior wall of the nasopharynx. However, it was subject to significant artifacts caused by breathing and swallowing. The sphenoidal electrode is an alternative that can be used to semiinvasively sample the anteromesial temporal lobe. It consists of a thin, Teflon-coated platinum or chlorided silver wire that is placed near the foramen ovale. Using sterile technique, the sphenoidal electrode is inserted with a 20 or 22 gauge spinal needle, 1cm anterior to the tragus, beneath the zygomatic arch and toward the foramen ovale, approximately 3 to 4 cm deep to the skin.
Invasive monitoring of cortical electrical activity is performed using depth electrodes or subdural strip or grid array electrodes. The depth electrode is a thin, flexible Teflon sheath having 6 or 8 concentric stainless steel or platinum contacts along it with interelectrode distances of 5 or 10 mm. It is placed stereotactically using a rigid introducer, which is removed after electrode placement Subdural strip or grid array electrodes consist of stainless steel or platinum discs embedded in a Silastic or Teflon sheet The electrode contacts are separated by distances typically measuring 1 cm. Subdural electrodes are placed through a craniotomy site, the size of which is determined by the size of the electrode strip or array. These invasive electrodes may be used either extraoperatively during video-EEG monitoring or intraoperatively during surgical excision. Their primary use is to more accurately define an epileptic focus. In addition, the cortical surface electrodes can be used to stimulate the surface of the brain to determine the function of a specific area of cortex, such as speech, language comprehension, or motor control.
| EEG Machine |
The cerebral electrical activity is conducted by wires from the scalp and/or invasive electrodes to the jackbox of the EEG machine. The inputs to the jackbox are then used to compose a montage, which is a specific arrangement or array of electrodes that display the EEG. The EEG machines currently available use 16, 18, or 21 channels. Each channel consists of a differential amplifier, which compares the input of two electrodes and amplifies the output to the pen-writing system or video display screen.
| An upward pen deflection is defined as negative, and occurs when input I is negative with respect to input 2 or when input 2 is positive with respect to input I. A downward pen deflection is defined as positive, and occurs when input 1 is positive with respect to input 2 or when input 2 is negative with respect to input 1. These two conditions are illustrated in Fig-2, where there is a downward deflection in channel 1 because input 2 is more negative than input 1, and an upward deflection in channel 2 because input 1 is more negative than input 2. This example demonstrates a surfacenegative phase reversal in a bipolar montage, which localizes maximal surface electronegativity. Depending on the polarity of each input, or electrode, there may be summation, no change, or cancellation of the cerebral EEG activity between the two inputs. Cancellation indicates a region of isoelectricity, and the result is no change in the output of the amplifier and no pen deflection.Before being displayed, the amplifier outputs are filtered, typically using a low-frequency filter setting of I Hz and a highfrequency filter setting of 70 Hz. A 60-Hz "notch" filter may be used in a recording environment with excessive electrical interference, such as an intensive care unit. The amplitude of each channel can be adjusted by changing the amplifier gain, or sensitivity. The amplified, filtered outputs are then displayed either with an analog pen writing system or digitally on a video monitor. The routine paper speed is 30 cm/s, so that each page of EEG displays 10 s of electrical cerebral activity. A paper speed of I5 cm/s is often used in neonatal EEGs in order to compress the EEG and accentuate focal slowing. A faster paper speed such as 60 cm/sec can be used to expand the time scale in an attempt to see if two potentials or events occur synchronously or to better define high-frequency activity. | 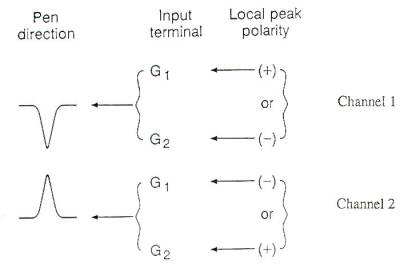 |
| Fig-2:Pen deflection based on inputs G1 and G2 into channels 1 and 2. |
| Montages |
The brain's electrical activity is logically displayed using different montages, which are specific arrangements of electrodes. Each montage provides the electroencephalographer with a different view, to delineate both normal and abnormal activity. The objective of any montage is to display the electrical field potentials generated by cortical neurons. The output from each channel in a montage represents the voltage difference of the inputs from each pair of electrodes into the differential amplifier.
Current standards specify that each montage attempt to maintain a linear arrangement of electrodes having equal interelectrode distances. The display is oriented from anterior to posterior and from left to right. A bipolar montage is constructed by linking successive electrodes into sequential channels. In a referential montage, each electrode is 'referred' to a reference electrode, such as the ipsilateral ear or the vertex (Cz). The most commonly used montages are the longitudinal (anterior to posterior, or AP) bipolar montage, the transverse (left to right) bipolar montage, and the referential (to the ipsilateral ear) montage.
Typically, bipolar montages are used to localize the region of an abnormality, This is often seen as a phase reversal between two or more electrodes in a given region. Referential montages are useful to define the field or distribution of the abnormality by the amplitude of electrocerebral activity at the electrodes in the region of interest. Both types of montage have their disadvantages, Bipolar montages are susceptible to field cancellation because adjacent electrodes may be isoelectric in potential. Also, if the region of interest lies at the end of the linear chain of electrodes, no phase reversal will be apparent. In referential montages, it is important to be aware that if the reference is located in the field of the cerebral electrical signal of interest (called an active reference), cancellation or reverse polarity may be seen in the channels to which uninvolved electrodes are referenced. Finding an uninvolved or inactive reference may be difficult.
| Obtaining the EEG |
Including the patient setup time, a routine EEG takes approximately 60 to 90 min and produces a 30-min recording. Electroencephalography on patients in the intensive care unit (lCU) or on neonates often takes longer, both because setting up takes longer and because a longer recording is made. The ICU is often a hostile environment for electroencephalography, owing to the abundance of electrical monitoring equipment, which may result in an excessive 60-Hz noise artifact on the EEG recording,
While placing the electrodes, the technologist obtains the patient's clinical history and past medical history, and a family history for epilepsy or clinical problems similar to those of the patient. Medications currently being taken are listed, especially ones that may affect the EEG, such as barbiturates, benzodiazepines, tricyclic antidepressants, or neuroleptic medications. Medication for sedation or sleep induction is also noted. If there is a skull defect from previous trauma or intracranial surgery, it is depicted diagrammatically on the front sheet of the EEG. At the beginning of the EEG recording, electrical and biological calibrations are performed. The sensitivity, high-frequency filter, time constant, or low-frequency filter, and the use of any other special filters (e.g., a 60-Hz notch filter) are also noted on the first page of each montage, as well as the level of consciousness and the mental state of the patient. Approximately 10 min of uninterrupted recording are performed for each montage. Longitudinal bipolar, transverse bipolar, and referential montages are obtained, and the technologist may also obtain additional montages to better display a suspected abnormality. The patient is allowed to fall asleep, and, later in the recording, attempts are made to fully alert the patient by testing memory or calculations. Also, during the recording, photic stimulation is performed to evaluate for photosensitive seizures. Last, the patient is asked to hyperventilate for 3 to 5 min in an attempt to accentuate focal slowing or focal or generalized epileptiform activity.
| EEG Terminology |
A standard terminology is used to consistently describe each EEG. These terms summarize the electrocerebral activity as well as any abnormal waveform or transient in each region of the brain during the EEG. These terms are frequency, amplitude, polarity, morphology, distribution, rhythmicity, synchrony, reactivity, and persistence. Each term will be briefly discussed below.
Frequency refers to the repetition rate or number of cycles per second (Hz) of a given waveform. The frequency of a single waveform can be calculated from the inverse of the peak-to-peak duration of the waveform (1/time). During periods when the EEG is relatively sinusoidal, the frequency can be estimated by counting the number of cycles per 1 second epoch. Four frequency bands appear in EEGs and have been named delta (0.5 to 3.5 Hz), theta (4.0 to 7.5 Hz), alpha (8.0 to 12.5 Hz), and beta (13 Hz and greater).
Amplitude is the magnitude of the EEG activity in microvolts (µV). It is determined by measuring the pen deflection in millimeters (mm) at a specified machine sensitivity (µV /mm). Most EEGs are performed at a sensitivity of 7 µV /mm, such that a 10 mm pen deflection signifies an amplitude of 70 µV. In describing the EEG, quantitative measures may be used (i.e., 50 to 70 µV), or a qualitative scale may be used, in which low amplitude is defined as less than 20 µV, medium amplitude as 25 to 95 µV, and high amplitude as greater than 100 µV.
Polarity is the sign of the EEG activity and may be negative, positive, or isoelectric (i.e., zero). By convention, upward pen deflection signifies negative polarity, and downward pen deflection signifies positive polarity.
Morphology refers to the shape of the EEG waveform. It may be regular (i.e., sinusoidal) or irregular, monophasic, or polyphasic (e.g., a triphasic wave). The morphology of a transient is essential to determining whether the transient is normal or abnormal, nonepileptiform or epileptiform.
Distribution of EEG activity may be focal or generalized. If focal, the activity should be defined by side and region involved (i.e., frontal, temporal, central, parietal, occipital, or midline). Generalized activity is widespread, involving both hemispheres equally. Although widespread, generalized activity is often either anteriorly or posteriorly predominant.
Rhythmicity: The EEG is rhythmic when it has a sinusoidal pattern at a relatively constant frequency. Arrhythmic activity is a mix of frequencies and morphologies.
Synchrony: EEG activity that occurs at the same time in different regions of the brain is called synchronous. Activity that occurs at the same time and same location on both sides of the scalp is bilaterally synchronous. or bisynchronous. Conversely, activity that occurs at different times is asynchronous.
Reactivity refers to alteration in the EEG activity caused by stimulation of the patient. This is accomplished by visual stimulation (opening and closing the eyes), noxious stimulation (pinching the patient), auditory stimulation (a loud noise), or cognitive stimulation (simple arithmetic calculations). An unreactive EEG is one that shows no variation in activity over all scalp leads despite vigorous attempts at stimulation.
Persistence: A specific EEG activity appearing in a given region of the brain can be either intermittent or persistent. A persistent activity is present in the region for at least 70 to 80 percent of the record, despite stimulation and state change. EEG activity that is present in the region for less than 70 to 80 percent of the record is called intermittent, and may be further designated as rare, occasional, or frequent, depending on its total amount in the record.
| Normal EEG |
The age of the patient and the level of consciousness (i.e., awake or asleep) are critical parameters in describing the normal EEG, as both factors determine the frequency, amplitude, polarity, morphology, distribution, rhythmicity, synchrony, reactivity and persistence of the activities that are recorded. The EEG of the neonate is significantly different from that of the infant of 3 months or older, and it will be discussed below.
In the normal awake EEG, the most notable feature is a posteriorly dominant, rhythmic activity that is symmetric, bisynchronous, and reactive. This activity has been called the alpha rhythm. which must be distinguished from the previously described alpha frequency range of 8 to 12.5 Hz. At approximately 3 months of age, a posteriorly dominant background alpha rhythm can be seen, which is high-amplitude, 3 to 4-Hz activity. From infancy through the early teenage years. the mean frequency of the background alpha rhythm increases gradually to 10 Hz. and the amplitude decreases to moderate voltage (Fig-3). These values then persist throughout adulthood and old age. Subharmonics (one-half normal frequency) and harmonics (twice normal frequency) of the alpha rhythm occur in a small percentage of normal individuals. These variants are reactive to various stimuli. A pattern of lowamplitude, mixed fast frequencies can also be seen in normal adults, which may be due in part to subjects' inability to relax adequately during the EEG. In the central head regions of adult subjects, the EEG consists of moderate to low-amplitude alpha and theta-range frequencies. whereas in the frontal head regions. lowamplitude beta-range frequencies are generally seen. These activities should be bisynchronous and symmetric. In 10 to 20 percent of young adults, either or both central head regions may show rhythmic, arciform alpha-frequency activity that is reactive to the patient performing mental arithmetic operations or moving the contralateral hand. This is called mu rhythm.
| 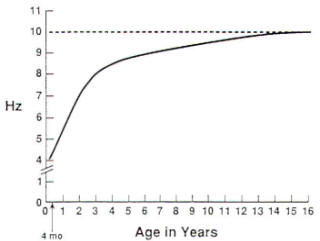 |
Fig-3: Background alpha rhythm and age relation.
|
Sleep has been divided into non-rapid-eye movement (NREM) and rapid-eye-movement (REM) phases. NREM has four stages: stage I (drowsy), stage II, stage III and stage IV. As the patient becomes drowsy, the background alpha rhythm becomes arrhythmic, with intermixed theta and beta frequencies that spread into the central head regions. A slow lateral eye movement artifact may be visualized on the EEG in the anterolateral head regions, because the retina is electronegative with respect to the cornea, resulting in an electrical dipole whose field changes with eye movement. Two additional features of stage I sleep are sharply contoured, surfacepositive theta transients of moderate amplitude that appear synchronously or asynchronously in the posterior head regions (positive occipital sharp transients of sleep or POSTS) and moderate to high-amplitude, sharply contoured, biphasic theta or alpha transients that phase-reverse at the vertex (vertex sharp waves). Stage II of sleep is defined by the presence of K complexes and sleep spindles. The K complex is a high-amplitude. biphasic slow wave of 0.5 to I s duration that has a distribution similar to that of the vertex sharp wave. The sleep spindle consists of rhythmic. moderate-amplitude alpha frequency activity lasting 0.5 to 1 s which is bisynchronous in the central head regions. In deeper stage I and stage II sleep, the remaining background consists of moderate to low-amplitude mixed theta, alpha, and beta frequencies. In stages III and IV of sleep, there is increasing delta activity having high amplitude and anterior predominance. In REM sleep, the EEG consists of diffusely distributed, moderate to low-amplitude mixed frequencies with rapid eye movement artifacts seen in the anterolateral head regions. The features of NREM sleep are absent during REM sleep (i.e., vertex sharp waves, sleep spindles, and K complexes).
| Benign Variants and Artifacts |
One of the major goals of EEG is to accurately define which EEG patterns are consistent with the diagnosis of seizures, and which patterns may be of no clinical significance (that is, normal). The "epileptiform" patterns and "seizure-like" discharges that are not significantly associated with seizures are called benign EEG variants and, in general, are considered normal findings on the EEG when it is properly obtained. For each of these patterns, the interpretation depends critically on the age and clinical state of the patient and the distribution, frequency, amplitude, and morphology of the waveform(s). The benign epileptiform patterns include benign epileptiform transients of sleep (BETS), 14 and 6-Hz positive bursts, 6-Hz spike and wave (phantom spike and wave), and wicket spikes. The benign seizure-like discharges include rhythmic midtemporal discharges (RMTD or psychomotor variant), midline theta rhythm, frontal arousal rhythm (FAR), and subclinical rhythmic electrographic discharges in adults (SREDA).
An EEG activity that does not originate from the brain is called an artifact. Artifacts can be divided into two major groups, physiologic and nonphysiologic. The accurate identification of artifacts can be crucial to the correct interpretation of both normal and abnormal EEGs. An electrically hostile environment such as an ICU often proves to be a significant challenge to the EEG technologist, who must recognize and, if possible, eliminate all artifacts. Any source in the body that has an electrical dipole or generates an electrical field is capable of producing a physiologic artifact. These include the heart (electrocardiogram and ballistocardiogram or pulse artifact), eyes (oculomotor artifact), muscles (myogenic artifact), and tongue (glossokinetic artifact). Sweating may alter the impedance at the electrode-scalp interface and produce an artifact. In the region of a skull defect, there may be accentuation of amplitude with very sharp morphology, which is called breach rhythm. Examples of nonphysiologic artifacts include 60-Hz interference from nearby electrical equipment, kinesiogenic artifacts caused by patient or electrode movement, IV drip artifact caused by a charged saline solution, and mechanical ventilator artifacts caused by patient movement or fluid movement in the ventilator tubing.
| Abnormal EEG |
Most abnormal EEG findings are defined by localizing the region of maximal electrode negativity associated with the abnormality. Models of radially oriented neurons have been proposed to define the origin of cortical electronegativity. However, much of the brain's cortical surface lies along the base and walls of sulci. Using scalp and cortical electrodes, Cooper and colleagues estimated that approximately 6 cm2 of cortical surface was necessary to generate scalp-recorded electrical potentials. Abraham and AjmoneMarsan have demonstrated that only 20 to 70 percent of spike discharges seen using electrocorticography are seen on scalp EEG. Significant abnormalities on the EEG consist of slowing, lack of reactivity, interictal epileptiform activity. periodic patterns, and ictal patterns. The clinical and pathologic importance of each finding depends on whether it is focal or generalized, intermittent or persistent. Although an amplitude asymmetry of greater than 50 percent is also considered abnormal, it must be demonstrated on multiple montages, including a referential montage, preferably to a common reference. Amplitude asymmetries are often the result of normal anatomic variations (e.g., in skull thickness) or technical factors (interelectrode distances. electrode impedances. etc.).
As noted above. the normal frequency range for the background alpha rhythm is from 8 to 12.5 Hz. Therefore, in a maximally alerted adult patient, a background alpha rhythm of less than 8 Hz is considered abnormal. Intermittent, generalized delta slowing may appear as isolated diffuse polymorphic delta transients or as rhythmic delta activity. Intermittent rhythmic delta activity (IRDA) may be seen having frontal predominance (FIRDA) in adults or occipital predominance (OIRDA) in children. These findings are nonspecific in etiology. However, each abnormality noted above is consistent with diffuse bihemispheric cerebral dysfunction. In adult patients, the severity of the cerebral dysfunction is related to the degree of theta or delta slowing of the posterior background frequencies or to the total amount of generalized delta slowing that occurs during the EEG record.
Persistent frequency asymmetries of greater than 1 Hz between corresponding scalp regions are abnormal. Focal slow transients in the delta range often have variable morphology (are polymorphic) and are considered abnormal in all fully alerted, adult patients, with the exception of rare dominant-hemisphere temporal delta slowing in the elderly. When focal delta slowing is present for 70 to 80 percent of the record, it is called persistent polymorphic delta activity, or PPDA. Although not specific in etiology, focal PPDA is consistent with a structural lesion in the absence of a recent transient neurologic event such as a seizure, transient ischemic attack, or complicated migraine headache. If focal polymorphic delta activity appears in less than 70 percent of the record, it is noted as intermittent and qualified as rare, occasional, or frequent, depending on the total amount seen during the EEG. Intermittent focal delta slowing is nonspecific in etiology and clinical significance and is thought to be consistent with focal neuronal or cerebral dysfunction in the region of the slowing.
Generalized loss of reactivity is evidence of severe diffuse bihemispheric cerebral dysfunction, regardless of the dominant frequency. This finding is commonly seen in coma and will be discussed in more detail below. Focal loss of reactivity may be seen in the setting of an intracerebral structural abnormality, such as a cerebral infarct, abscess, or tumor. Focal unreactivity of the posterior background rhythm with eye opening is called Bancaud's phenomenon, because of its location, it is the most readily recognized form of focal unreactivity.
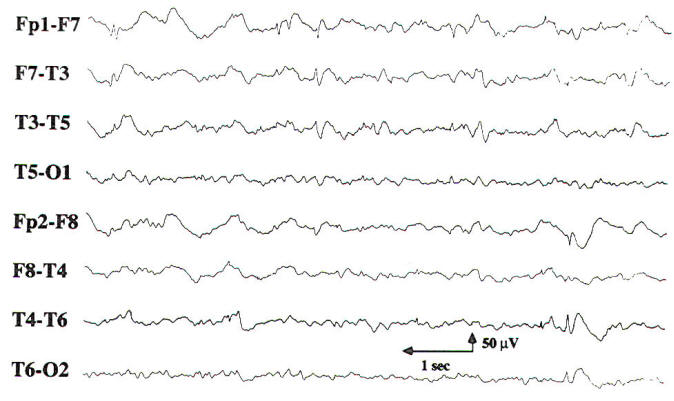 | 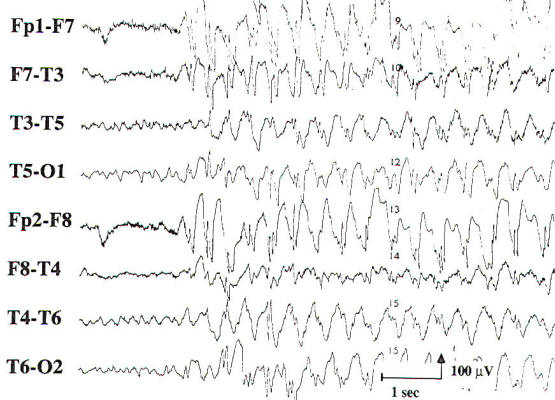 |
| Fig-4: Bilateral independent temporal spikes and sharp waves. Left side greater than right. | Fig-5: Burst of generalized 3-Hz spike and wave activity. |
Two transients of special interest are the sharp wave and the spike discharge. These transients are important because of their high correlation with seizures, and they are often referred to as epileptiform discharges. They are defined by their morphology and duration, with sharp waves having a duration of 70 to 200 ms and spike discharges having a duration of 20 to 70 ms. When accompanied by an after-going slow wave, they are referred to as a sharp or spike and slow wave complex. If an epileptiform discharge appears focally, it is localized by finding a region with a phase reversal on the bipolar montages (Fig-4). Using a referential montage with an uninvolved reference. a focal epileptiform discharge is localized by defining the region of greatest electronegativity. The generalized epileptiform transient is most commonly a spike discharge, which may appear as an isolated spike, a spike-wave complex, or a polyspike and wave complex. Although they are called generalized, these discharges are often anteriorly predominant and may have shifting left or right sided emphasis, which averages out during the EEG. Spike-wave and polyspike-wave complexes often appear as repetitive discharges having a predominant frequency based on the repetition rate of the discharges (Fig-5 ).
The clinical significance of the epileptiform discharge will be discussed in more detail later. It must be properly recognized and distinguished from benign variants, artifacts, and normal EEG activity. Focal delta slowing in the same region as a suspected epileptiform discharge is additional evidence for focal neuronal dysfunction in the region of the presumed epileptic focus.
Sharp waves and spikes generally occur intermittently. In certain clinical settings, such as acute hemispheric cerebral infarction, sharp waves appear in a periodic fashion, and they are then referred to as periodic lateralized epileptiform discharges (PLEDs). PLEDs are most often seen in acute structural brain lesions. Generalized periodic sharp wave activity is classified on the basis of its morphology and frequency along with the specific clinical presentation. Examples include triphasic waves, generalized periodic epileptiform discharges (GPEDs), and the burst suppression pattern. Each of these patterns indicates that there is severe diffuse cerebral dysfunction.
An epileptic seizure rarely occurs during an EEG. The hallmark features of an ictal pattern are an evolution in frequency and field of the EEG activity during the event. Evolution in frequency refers to an increase or decrease from the initial frequency: evolution in field refers to spread of the activity into adjoining regions. The amplitude of the activity may increase, decrease, or remain the same during the ictal pattern or discharge. When there is an accompanying change in the clinical state of the patient, these findings are diagnostic of a seizure disorder. Ictal patterns that are not accompanied by a clinical change in the patient are called subclinical seizures. If the ictal discharge is focal in onset, then the seizure disorder is said to be partial in origin. However, if the discharge is generalized at onset, the seizure disorder may be either generalized or partial in origin, as a focal midline seizure focus may project equally to both hemispheres with a wide field. The morphology of the discharges, age of the patient, and ictal semiology are important factors in defining the type of seizure.
Activation procedures are used to enhance or increase abnormalities on the EEG. Although focal slowing is the abnormality most likely to be "activated," these procedures may also accentuate epileptiform activity or induce a seizure. Activation procedures currently in use consist of hyperventilation (HV), intermittent photic stimulation (IPS), spontaneous and medicationinduced sleep, and sleep after sleep deprivation. In the past, injections of seizure-inducing drugs such as pentylenetetrazol were used during the EEG to activate spike foci and induce seizures. However, these techniques are no longer used owing to the risk to the patient and the difficulty of discriminating spontaneous from drug-induced interictal and ictal discharges.
Hyperventilation should be performed for 3 to 5 min by any cooperative patient at least once during the EEG, provided there are no medical contraindications (cardiopulmonary disease, unstable cerebrovascular disease, etc.). Focal delta slowing that has been noted during wakefulness or drowsiness is often accentuated during hyperventilation. The induction of typical 3-Hz generalized spike and wave discharges and absence seizures by hyperventilation is well known. Hyperventilation has also been found to activate focal epileptiform discharges much less often than generalized epileptiform discharges. If hyperventilation provokes an absence seizure in a patient with an idiopathic generalized epilepsy, clinical unresponsiveness should be confirmed during the ictal discharge. Similar testing should also be performed in patients suspected of having nonepileptic seizures, or pseudoseizures, because hyperventilation may provoke a nonepileptic seizure in such patients.
Stimulus frequencies used during intermittent photic stimulation range from 1 to 20 Hz in increments of 2 to 3 Hz. In most subjects, a posteriorly predominant, bisynchronous and timelocked "driving response" is seen normally. The responses are best seen in the lower range flash frequencies in the very young and in the midrange frequencies in the adult. The absence of a driving response is also normal. In some subjects, the driving response may appear "spiky." Other normal findings during intermittent photic stimulation include the electroretinogram (ERG), which is seen in the frontopolar leads, and the photomyoclonic response (PMR), which is a synchronous myoclonic response involving the patient's facial and neck musculature, resulting in myogenic and kinesiogenic artifacts on the EEG. The artifacts generated by the PMR may appear as generalized spike or spike and wave activity. The PMR must be differentiated from the photoparoxysmal response (PPR), which is a burst of generalized epileptiform activity that is evoked synchronously by the intermittent photic stimulation, typically in the midrange frequency in susceptible patients, PPR may be seen in patients with an idiopathic generalized epilepsy, such as juvenile myoclonic epilepsy or absence epilepsy.
The process of becoming drowsy (stage I sleep) and falling into deeper stages of sleep has been shown to activate interictal epileptiform discharges of both focal and generalized types. This is accomplished in the EEG laboratory by recording during spontaneous sleep or sleep induced by medications (e.g., chloral hydrate). Typically, the epileptiform discharges appear more frequently on a scalp EEG during the lighter stages of sleep (stages I and II), and they appear less frequently during deeper stages of NREM sleep (stages III and IV) and during REM sleep. However, by using depth electrodes in patients with intractable partial seizure disorders, Rossi and colleagues demonstrated that interictal epileptiform discharges increase in frequency with increasing depth of NREM sleep.
The effect of sleep deprivation is less well established. Although it is often used as an activating method in patients with suspected seizures after a routine EEG without epileptiform features, it is not clear whether it causes any activation of the interictal epileptiform activity beyond that caused by falling asleep. Nevertheless, many EEG laboratories continue to recommend sleep deprivation with sleep as a follow-up EEG after a nondiagnostic routine EEG.
| Specific Clinical Applications |
Although EEG is used most often as an ancillary test in clinical epilepsy, it also is an invaluable tool in other neurological conditions, such as encephalopathy, focal central nervous system (CNS) lesions, and clinical brain death, as well as for electrocorticography, and in neonatal medicine. The following sections discuss the usefulness of EEG in each of these situations.
| Epilepsy |
Epilepsy is defined as "paroxysmal transient disturbances of brain function that may be manifested as episodic impairment or loss of consciousness, abnormal motor phenomena, psychic or sensory disturbances, or perturbation of the autonomic nervous system. A seizure, or ictus epilepticus, is an epileptic attack or recurrence. The classification of epilepsies used by International League Against Epilepsy (ILAE) includes two major categories: partial epilepsies and generalized epilepsies. A partial seizure disorder is considered to have a focal region of onset in the brain, and awareness may be either preserved (simple partial seizure) or lost (complex partial seizure). A generalized seizure disorder is considered to involve most, if not all, of the brain at onset. The generalized seizure types may involve cessation of activity with loss of awareness (absence seizure) or generalized tonic-clonic activity (generalized tonic-clonic seizure). Both partial and generalized seizure disorders are further subdivided into idiopathic and symptomatic types, previously called primary and secondary, respectively. Idiopathic epilepsies are thought to be genetically heritable, are associated with normal intelligence, and occur during specific age periods. The symptomatic epilepsies are likely the result of a CNS injury, which in a symptomatic partial epilepsy consists of a focal lesion and in a symptomatic generalized epilepsy consists of diffuse cerebral abnormality. Symptomatic epilepsies are typically lifelong conditions.
It cannot be overemphasized that the diagnosis of epilepsy is based primarily on the clinical history. As noted above, a clinical seizure rarely occurs during an EEG, and thus the EEG is rarely diagnostic of a seizure disorder or epilepsy. In a large, populationbased EEG study by Zivin and Ajmone-Marsan involving subjects without a history of seizures, approximately 2 percent of the subjects had EEGs with epileptiform discharges. Of the individuals in this subgroup, only 15 percent subsequently developed a seizure disorder. Therefore, epileptiform discharges seen on an EEG should not be referred to as interictal discharges unless it is known that the patient has a clinically defined seizure disorder. Focal or generalized epileptiform discharges should be noted as consistent with the interictal expression of either a partial or a generalized epilepsy, respectively. When applied in the appropriate clinical setting, the EEG is useful in classifying the seizure type, predicting the long-term outcome, and choosing the appropriate antiepileptic medication.
Overall, symptomatic partial seizure disorders are the most common type of epilepsy. The clinical semiology of the partial seizure generally depends on the site of onset. In children, focal epileptiform discharges arising from the temporal region have the greatest incidence of clinical seizures, ranging from 85 to 95 percent. The next highest incidence (70 to 75 percent) is associated with frontal discharges. The central, parietal and occipital regions have the lowest incidence of seizures related to epileptiform discharges. estimated at 40 to 70 percent. In addition to the characteristics of recorded epileptiform activity, the age of the patient and the presence or absence of neurological deficits on examination are important factors that are helpful in determining the clinical significance of epileptiform discharges and in classifying the partial seizure disorder as either symptomatic or idiopathic. The occurrence of a clinical seizure with a focal electrographic correlate is diagnostic of a partial epilepsy. Blume and colleagues presented several types of scalp EEG correlates for partial seizures, most of which began with rhythmic sinusoidal activity or repetitive sharp wave activity that subsequently evolved in frequency. Most patients with complex partial seizures were noted to have a scalp correlate on the EEG. Patients with simple partial seizures were less likely to have a scalp correlate.
The best-defined idiopathic partial epilepsy is benign rolandic epilepsy. The classic EEG finding in this childhood seizure disorder is a characteristic monomorphic centrotemporal sharp wave. The sharp waves are often seen independently in the centrotemporal and adjacent regions, and they are accentuated by light sleep. The waking background rhythm is generally normal.
Of the idiopathic generalized epilepsies, the absence seizure is the most common type. The interictal EEG feature of this type of seizure disorder consists of generalized, high-amplitude, anteriorly predominant 3-Hz spike and wave discharges, called typical 3-Hz spike and wave. When the spike and wave discharges occur repetitively, they are called bursts. Although these discharges are called "3-Hz." the initial frequency of the burst is 3 to 4 Hz, and the frequency may slow to 2.5 Hz during more prolonged bursts. The discharges are reactive to alerting maneuvers and may become fragmented in deeper stages of sleep. Juvenile myoclonic epilepsy (JME) is another type of idiopathic generalized epilepsy. The spike and wave discharges of this seizure disorder are also generalized and anteriorly predominant, but they have an initial frequency 0f 4 to 6 Hz and may begin with a polyspike discharge. The EEG of a patient with an idiopathic generalized epilepsy who is maximally alerted is generally normal. During photic stimulation, there may be a photoparoxysmal response in both absence epilepsy and JME, which may be helpful in classifying recognized epileptiform discharges as consistent with an idiopathic generalized epilepsy rather than a symptomatic partial or generalized epilepsy.
Epileptiform patterns in symptomatic generalized epilepsies are of three types. A slow spike and wave pattern at approximately 2 Hz is seen in patients with mental retardation having multiple seizure types (atypical absence, tonic, atonic, or tonic-clonic seizures), which is known as the Lennox-Gastaut syndrome. A second type of interictal or ictal EEG pattern seen in patients with symptomatic generalized epilepsy is generalized paroxysmal fast activity (GPFA), which consists of bursts of rhythmic, generalized beta activity. When the bursts are seen during wakefulness, they are commonly accompanied by a tonic seizure. During sleep. bursts of GPFA not accompanied by clinical changes are considered an interictal pattern. The third pattern of epileptiform activity in secondary generalized epilepsy is an atypical generalized spike and wave pattern, consisting of generalized 3 to 6-Hz spike or polyspike and wave activity. The waking background in patients with secondary generalized epilepsies is abnormally slow, including slowing of the posterior background rhythm and generalized slowing.
In patients suspected of having a seizure disorder, a normal routine, awake EEG should be followed with either a natural or medication-induced sleep EEG or a sleep-deprived EEG. Before the advent of long-term video-EEG monitoring for the diagnosis of possible seizures, three or more EEGs were often obtained to confidently conclude normality and absence of epileptiform activity. Because antiepileptic medications have been shown not to affect the frequency of focal interictal epileptiform discharges, the decision to treat a patient for a suspected partial seizure disorder should not be based solely on the initial EEG findings. Conversely, the EEG has not proven to be a reliable tool in predicting whether a patient's antiepileptic medication can be discontinued. In patients with an idiopathic generalized epilepsy, treatment with appropriate antiepileptic medication may eliminate all interictal epileptiform activity on the EEG. Therefore, the decision to discontinue an antiepileptic medication in a patient with a seizure disorder should be based on the type, etiology and response to medications of the seizures and not on interictal EEG findings.
| Encephalopathy and Coma |
Encephalopathy and coma result from conditions that affect both cerebral hemispheres or the reticular activating system in the midbrain. The differential diagnosis is broad, including metabolic, toxic, anoxic/ischemic, infectious, endocrinologic, degenerative, and inflammatory processes. These processes affect the brain diffusely, and, consequently, changes in the EEG often appear generalized. While most EEG findings in encephalopathy and coma are nonspecific with regard to etiology, information relevant to the clinical course and prognosis can be obtained using the EEG.
In cases of mild encephalopathy, theta and delta activity is intermixed with the background alpha rhythm. Occasional generalized delta transients are also seen. As the encephalopathy worsens, there is loss of background alpha-range frequencies and an increased amount of generalized theta and delta activity. Intermittent-rhythm delta activity (IRDA) may appear, which in adults generally is frontally predominant (FIRDA), and is consistent with moderate diffuse bihemispheric cerebral dysfunction (Fig-6). In severe encephalopathy, there is generalized delta activity. Loss of reactivity in anyone of these stages implies greater severity, and, in specific clinical settings, a worse prognosis. In the clinical setting of severe anoxia (e.g., after cardiac arrest) or severe closed head injury, invariant patterns of persistent, generalized alpha activity (alpha coma), generalized periodic epileptiform discharges, or the burst suppression pattern (Fig-7) are associated with very poor outcome.
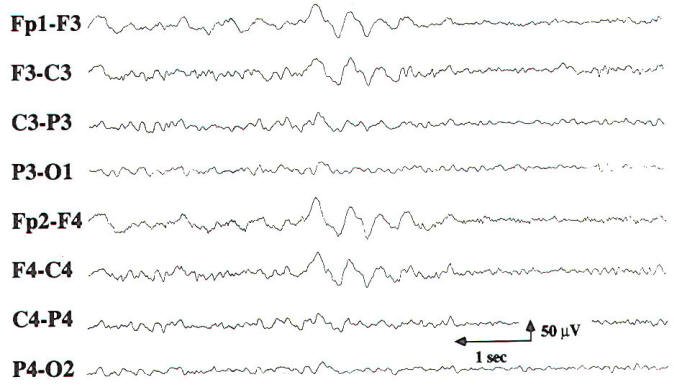 | 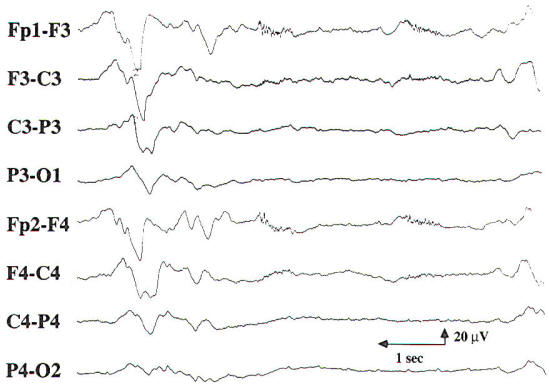 |
| Fig-6: Frontal intermittent rhythmic delta delta activity (FIRDA) | Fig-7: Burst suppression pattern, consisting of bursts with an initial delta transient and superimposed theta activity lasting 2 s. During the burst intervals, there is no EEG activity. Ventilator artifacts are seen. |
In the early reports of the EEG findings in hepatic coma, triphasic waves were noted which were initially thought to be pathognomonic for this condition. These three-phased generalized discharges consist of high-amplitude, sharp wave complexes that are repetitive, have an average frequency of 2 Hz, and show initial surface positivity and anterior predominance (Fig-8).
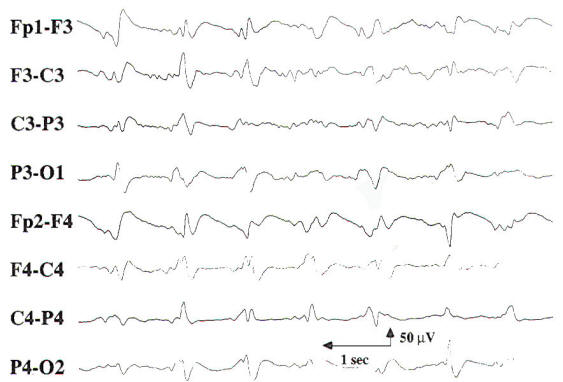 | |
Fig-8: Periodic triphasic waves at 1-Hz frequency.
|
Triphasic waves may be intermittent and reactive, or they may be persistent and unreactive. There is no normal background rhythm. Although present on the EEG in most patients with hepatic failure, triphasic waves may also be seen in cases of other metabolic, toxic, anoxic, degenerative and inflammatory encephalopathies. In patients whose EEGs demonstrate triphasic waves, overall mortality is high, and there are few normal survivors. Periodic sharp waves having a morphology similar to that of triphasic waves may be seen in patients with Creutzfeldt-Jakob disease (CJD), but the frequency of the discharges typically averages 1 Hz. In early CJD, the periodic complexes are superimposed on a background that may have only mild slowing. As the disease progresses, the background rhythm is lost, resulting in a pattern of periodic 1-Hz discharges on a flat background. The clinical history of subacute dementia, seizures, and myoclonus in conjunction with this periodic pattern is strongly suggestive of CJD. To confirm this progression, sequential EEGs may need to be performed during the course of CJD.
Epileptiform activity may be seen on the EEG in some degenerative encephalopathies that have associated seizures. Multifocal, independent epileptiform spike discharges may be seen in TaySachs disease, in several of the progressive myoclonic epilepsies (neuronal ceroid lipofuscinosis, Lafora body disease, and some mitochondrial encephalomyopathies), and in Rett syndrome. Atypical generalized spike and wave activity is present in UnverrichtLundborg disease, which is another type of progressive myoclonic epilepsy.
Of the inflammatory encephalopathies, distinctive EEG findings are seen in subacute sclerosing panencephalitis (SSPE) and herpes simplex encephalitis. The clinical presentation of SSPE includes myoclonus with progressive encephalopathy. The EEG shows periodic, polyphasic sharp and slow wave complexes that have an interburst interval of 4 to 10 s. As SSPE progresses, there is gradual loss of the intermixed background frequencies, resulting in a pattern similar to burst suppression. Herpes simplex encephalitis is the most common sporadic viral encephalitis, typically presenting with fever, encephalopathy, and secondarily generalized seizures. The EEG commonly shows periodic lateralized epileptiform discharges (PLEDS). which are lateralized to the side of the herpes infection. Should both temporal lobes be involved, bilateral independent periodic epileptiform discharges (BIPLEDS) may be seen on the EEG. Other forms of inflammatory encephalopathy typically result in nonspecific slowing of the EEG, the severity of which is often correlated with the severity of the encephalopathy.
Lastly, nonconvulsive status epilepticus should be considered in patients with a known seizure disorder or recently witnessed seizure who present with prolonged encephalopathy. Patients presenting in nonconvulsive status epilepticus may have subtle clinical findings of ongoing seizures, and electroencephalography is crucial in confirming response to therapy with cessation of electrographic seizure activity. The EEG in nonconvulsive status epilepticus generally shows widespread, repetitive sharp and slow wave complexes at 1 to 2 Hz. Administration of low-dose intravenous benzodiazepine therapy during the EEG usually results in rapid resolution of the ictal pattern and clinical encephalopathy. Should convulsive seizure activity not respond to conventional therapeutic intervention, then barbiturate coma or general anesthesia with concurrent EEG monitoring is needed to demonstrate a burst suppression pattern and lack of electrographic seizure activity.
| Focal Lesions of the Central Nervous System |
The EEG findings in focal cerebral lesions are generally nonspecific. Serial EEGs may be necessary to fully appreciate the electrographic changes in conditions where there may be significant change in neurological status, such as acute stroke or progressive brain tumor. If only the cortical gray matter is involved, there is amplitude suppression of the surrounding EEG activity. However, many focal cerebral lesions involve both the cortical gray matter and the underlying white matter, resulting in slowing of the EEG activity with intermittent focal delta activity. Midline and infratentorial lesions may not produce any changes in the EEG, or they may result in generalized slowing.
When focal delta activity is intermittent, it is consistent with focal cerebral dysfunction of a nonspecific etiology. Focal delta activity that is nonreactive and is present for 70 to 80 percent of the record (Fig-9) is called persistent polymorphic delta activity (PPDA). PPDA is a specific finding in structural lesions of the brain, often seen in patients with a supratentorial high-grade cerebral neoplasm, a large cerebral abscess, or a stroke involving subcortical and cortical regions. Transient PPDA may be seen after a complicated migraine headache or a partial seizure. emphasizing the need for serial EEGs in certain cases.
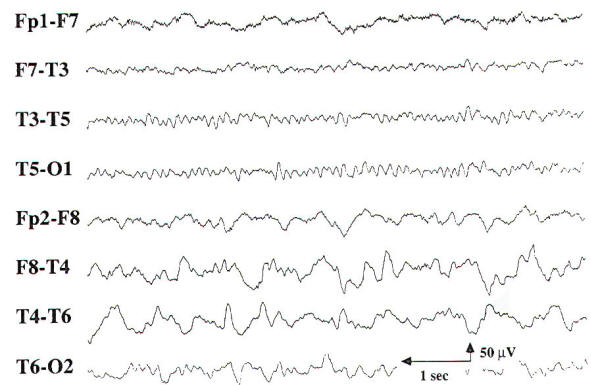 | |
| Fig-9: Persistent polymorphic delta activity (PPDA) in the right temporal region. |
As discussed above, intermittent rhythmic delta activity (lRDA) is generally a finding consistent with diffuse bihemispheric cerebral dysfunction. However, in a large series of patients with IRDA, brain tumor was seen in 30 percent and cerebrovascular disease in 19 percent. Although reported before the advent of computed tomography (CT), the diagnoses in this study were based on neuropathologic confirmation. The frontal lobe is the most common location for brain tumors associated with IRDA on the EEG.
Sharp waves or spike discharges are occasionally seen on the EEGs of patients with focal cerebral lesions. The epileptiform discharges are rarely the sole abnormality on the EEG, and they are most often associated with focal delta slowing. Periodic lateralized epileptiform discharges (PLEDs) may be seen in acute cerebral lesions such as stroke or herpes simplex encephalitis. PLEDs may be unilateral or bilaterally independent, termed BIPLEDs (Fig-10). PLEDs are generally self-limited, lasting 1 to 2 weeks during the acute phase of illness. There is a high incidence of seizures in patients whose EEG demonstrates PLEDs or BIPLEDs (Fig-11). Last, patients with a focal cerebral lesion may present in partial or generalized status epilepticus.
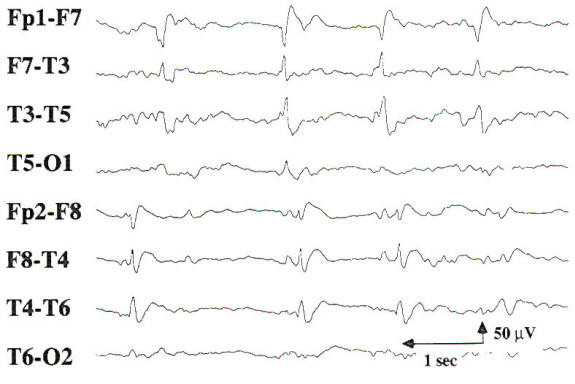 | 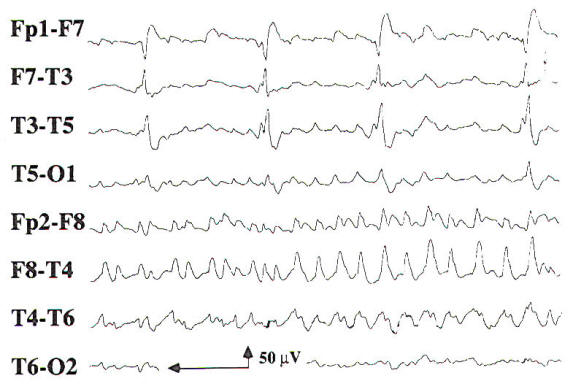 |
| Fig-10: Bilateral independent periodic lateralized epileptiform discharges (BIPLEDs) due to right ICH and IVH. | Fig-11: Focal seizure in right temporal region with ongoing PLEDs in the left temporal region. |
| Brain Death |
Brain death has been defined as the "irreversible cessation of all functions of the entire brain, including the brainstem." The determination of brain death is important in clinical situations such as potential organ donation and withdrawal of life support. The clinical criteria for brain death in adult patients can be summarized as follows:
1. There is no known reversible etiology. Reversible factors that may cause coma or apparent coma must be ruled out, including sedative medications and paralytics (e.g., barbiturates, benzodiazepines, neuromuscular blocking agents), hypothermia (i.e., the core temperature must be greater than 32.2°C), a potentially reversible medical illness (e.g.. hepatic failure, renal failure), and shock.
2. Coma and the absence of brain stem function (e.g., cranial nerve function and respiratory control) are demonstrated by a neurologist or neurosurgeon on two successive neurological examinations separated by an appropriate period. In adult patients, 12 h is generally an adequate period between examinations. However, in adults with anoxic/ischemic encephalopathy and in children, this interval may extend to 24 h or longer, depending on the circumstances. Criteria for newborns are not well established.
3. Confirmatory tests (e.g., EEG, cerebral angiogram or nuclear cerebral blood flow scan) may be used if the period of observation is less than that recommended above, as in the setting of organ donation. In all other circumstances, these tests are considered optional and are used at the discretion of the attending physician.
An EEG recording to determine brain death should not be considered until the clinical criteria are met. The EEG then be ordered to confirm electrocerebral inactivity or silence. (ECI and ECS. respectively). ECI is defined as lack of EEG activity greater than 2 µV. The following guidelines for performing an EEG to confirm ECI have been recommended by the American Electroencephalographic Society:
1. At least 8 scalp electrodes should be used, covering the frontal, central, temporal, and occipital regions of both hemispheres.
2. Interelectrode impedances should be between 100 and 1000 Ω.
3. The integrity of the recording system should be confirmed at the beginning of the recording. This is generally done by touching each electrode in succession and documenting the resulting electrode artifact.
4. Interelectrode distances should be 10 cm or greater.
5. Sensitivities must be increased from 7 µV /mm to 2 µV /mm during the recording, the duration of which should be at least 30 min, excluding time for EEG machine preparation (i.e., machine calibration at all sensitivities).
6. Filter settings should be 1 Hz for the low-frequency filter and 30 Hz or greater for the high-frequency filter.
7. Monitoring of additional cerebral and noncerebral sites should be done as needed. This is done to confirm the source of suspected artifacts, such as electrocardiogram, respiration. electromyogram. etc.
8. Unreactivity of the EEG should be documented using visual stimulation, auditory stimulation and somatosensory stimulation below and above the neck.
9. The EEG recording during ECI should be performed by a qualified technologist.
10. The EEG should be repeated if there is any doubt regarding the diagnosis of ECI.
It is crucial to remember that the EEG is only a confirmatory test for the presence of cerebral death and that the primary criteria are clinical. As the EEG is subject to artifacts whose source may not be determined, the utility of this test for confirmation of ECI may be limited in settings where factors which cause EEG artifacts are prevalent.
| Electrocorticography |
Electrocorticography (ECoG) is the technique by which the brain's electrocerebral activity is directly measured using either depth electrodes, cortical surface contact electrodes, or subdural electrode strips or arrays. Although ECoG is not a routine procedure, it has become widely used in the presurgical evaluation of patients with medically intractable partial epilepsy where the site of seizure onset cannot be adequately localized using noninvasive methods. ECoG has also proved to be an important technique for functional brain mapping of eloquent cortex during the neurosurgical resection of lesions such as brain tumors or vascular malformations.
In patients undergoing invasive monitoring with depth or subdural electrodes for epilepsy surgery evaluation, the decision of where to place the electrodes is based on several factors. including ictal semiology, interictal epileptiform activity and neuroimaging findings. Electrodes should be placed to cover the region of suspected seizure onset. Often the corresponding contralateral cortex is also covered, for reference and to confirm that there is a single zone of epileptogenesis. A sufficient number of seizures are recorded with video-EEG monitoring using the invasive electrodes, and the behavioral onset of seizures is timed to confirm that it follows the electrographic onset. Interictal epileptiform activity is much more often recorded when invasive electrodes are used, and it is often multifocal. Upon completion of monitoring, depth electrodes may be removed at the patient's bedside. Removal of subdural electrodes is generally performed in the operating room.
The primary use of cortical stimulation is to identify areas of essential cortex, such as those subserving motor, sensory or language function. Brain mapping using cortical stimulation may be performed during epilepsy surgery or other neurosurgical procedures such as the resection of tumors or vascular malformations. It may be conducted intraoperatively with the patient awake in the operating room, or extraoperatively in the patient's room, where testing may be performed over several days in sessions lasting 1 to 3 h as needed. At most centers, stimulation consists of 0.3 to 1 ms biphasic square wave pulses at 50 Hz, lasting from 2 to 5 s each. The stimulation intensity starts at 0.5 to 1 mA and is raised in increments of 0.5 to 1 mA until a neurological deficit is produced or afterdischarges occur or a maximum 15 mA stimulus intensity is reached. During dominant temporal lobe surgery, object naming alone may be used if the zone of resection is more than 2 cm distal to the defined language cortex. However, when the zone of resection must border on language cortex, more extensive testing is performed, including reading, repetition, naming and comprehension. Motor and sensory areas may be similarly mapped by evoking either muscle contraction when stimulating areas of the precentral gyrus or regions of paresthesia when stimulating the postcentral gyrus. In all cases, the lowest stimulation intensity that evokes a response should be used, to limit the current field to the region of interest.
Clinical seizures provoked by cortical stimulation are in general not predictive of the zone of epileptogenesis in patients with intractable partial seizures. Afterdischarge potentials are brief, self-limited electrographic seizures that may be produced by cortical stimulation. Afterdischarges may evolve into a clinical seizure, and for this reason, anticonvulsant levels in patients with partial seizures are maintained in the therapeutic range when cortical stimulation is being performed. At some medical centers, cortical stimulation is performed in an attempt to induce typical auras that the patient may experience. Temporal lobe epilepsy has been reported to have the highest concordance between spontaneous seizures and induced auras or seizures.
| Neonatal EEG |
The neonatal period extends from birth (including premature birth) to age 2 months. The conceptual age (CA), or age since conception, is an important factor in interpreting the neonatal EEG, because it defines the level of maturation of the CNS. As the premature brain matures, well-defined patterns are seen that help to differentiate normal from abnormal EEGs at specific ages. Owing to the small size of the neonatal head, the International 10-20 System of electrode placements is modified to allow for coverage of the frontal, central, temporal, and occipital regions. Physiologic parameters such as heart rate, respirations, eye movements and limb myogenic activity are also monitored to help differentiate active sleep (rapid eye movements, variable heart rate and respiration) from quiet sleep (no movement with regular heart rate and respirations), and wakefulness from sleep. The EEG is performed at a paper speed of 15 mm/s (one-half the adult paper speed). This is done to allow a longer sampling time (generally 1 h) and to compress the EEG, as it consists predominantly of theta and delta range frequencies. Above a CA of 48 weeks, the standard EEG is performed.
Before 22 weeks CA there is no discernible electrocerebral activity. As the neonatal brain matures, a discontinuous, invariant pattern is seen initially, which is gradually replaced by more continuous, variant patterns as term gestation is reached. At 26 weeks CA, a discontinuous pattern is seen, with bursts of high-amplitude, sharply contoured theta activity, which is maximal in the temporal regions. During the interburst periods, there is no discernible EEG activity. The EEG is unreactive and remains so until 34 weeks CA. By 30 weeks CA, active sleep can be distinguished from quiet sleep by a reduction in amplitude and the amount of delta activity. At this age, beta-delta complexes (delta brushes) are seen in both stages of sleep; they gradually become less frequent and disappear by term. By 35 weeks CA, the EEG is reactive. Independent frontal sharp transients are seen, as well as occasional equally distributed independent sharp transients in both hemispheres, which may be seen until term. Wakefulness can be distinguished from sleep at 36 weeks CA, demonstrating continuous, low-amplitude mixed frequencies. At term, the EEG should be synchronous and reactive and should demonstrate both active and quiet sleep and wakefulness.
The most common abnormality in a neonatal EEG is the absence or delayed appearance of normal patterns. A low-amplitude EEG may be due either to cerebral dysfunction or to an extracerebral fluid collection such as scalp edema or a subdural hematoma. An increased number of multifocal independent sharp transients indicates diffuse cerebral dysfunction, which is maximal in the region of the most frequent transients. These sharp transients are not called sharp waves or spikes, as they do not indicate a seizure disorder in the neonate.
The clinical and EEG findings of a seizure in the neonate vary in semiology and pattern, and some are controversial. Seizures in a neonate may not be accompanied by an electrographic correlate. Conversely, electrographic seizures may have no clinical correlate, or they may have subtle correlates such as apnea or heart rate changes. The EEG diagnosis of a seizure is based on the evolution of frequency of focal rhythmic activity, which may be limited to a single electrode. Generalized tonic clonic seizures very rarely are seen, likely because the myelination and dendritic arborization of the CNS is limited at this age.
Obtaining an EEG should be considered in all premature or term neonates who have evidence of significant neurological dysfunction on clinical examination. In addition to providing information regarding CNS maturation, the neonatal EEG is often helpful in guiding neuroimaging assessment by cranial ultrasound, CT, or MRI. As neonatal seizures may have subtle or no clinical manifestations, the EEG is an invaluable tool in the clinical evaluation of the neonate.
Hiç yorum yok:
Yorum Gönder